当前位置:
X-MOL 学术
›
BBA Biomembr.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Polarity of the ATP binding site of the Na+,K+-ATPase, gastric H+,K+-ATPase and sarcoplasmic reticulum Ca2+-ATPase.
Biochimica et Biophysica Acta (BBA) - Biomembranes ( IF 3.4 ) Pub Date : 2019-11-29 , DOI: 10.1016/j.bbamem.2019.183138 K R Hossain 1 , X Li 1 , T Zhang 2 , S Paula 2 , F Cornelius 3 , R J Clarke 4
Biochimica et Biophysica Acta (BBA) - Biomembranes ( IF 3.4 ) Pub Date : 2019-11-29 , DOI: 10.1016/j.bbamem.2019.183138 K R Hossain 1 , X Li 1 , T Zhang 2 , S Paula 2 , F Cornelius 3 , R J Clarke 4
Affiliation
|
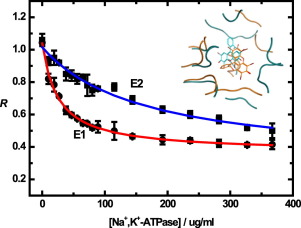
A fluorescence ratiometric method utilizing the probe eosin Y is presented for estimating the ATP binding site polarity of P-type ATPases in different conformational states. The method has been calibrated by measurements in a series of alcohols and tested using complexation of eosin Y with methyl-β-cyclodextrin. The results obtained with the Na+,K+-, H+,K+- and sarcoplasmic reticulum Ca2+-ATPases indicate that the ATP binding site, to which eosin is known to bind, is significantly more polar in the case of the Na+,K+- and H+,K+-ATPases compared to the Ca2+-ATPase. This result was found to be consistent with docking calculations of eosin with the E2 conformational state of the Na+,K+-ATPase and the Ca2+-ATPase. Fluorescence experiments showed that eosin binds significantly more strongly to the E1 conformation of the Na+,K+-ATPase than the E2 conformation, but in the case of the Ca2+-ATPase both fluorescence experiments and docking calculations showed no significant difference in binding affinity between the two conformations. This result could be due to the fact that, in contrast to the Na+,K+- and H+,K+-ATPases, the E2-E1 transition of the Ca2+-ATPase does not involve the movement of a lysine-rich N-terminal tail which may affect the overall enzyme conformation. Consistent with this hypothesis, the eosin affinity of the E1 conformation of the Na+,K+-ATPase was significantly reduced after N-terminal truncation. It is suggested that changes in conformational entropy of the N-terminal tail of the Na+, K+- and the H+,K+-ATPases during the E2-E1 transition could affect the thermodynamic stability of the E1 conformation and hence its ATP binding affinity.
中文翻译:

Na +,K + -ATPase,胃H +,K + -ATPase和肌浆网Ca2 + -ATPase的ATP结合位点的极性。
提出了一种利用探针曙红Y的荧光比率法,用于估计不同构象状态下P型ATP酶的ATP结合位点极性。该方法已通过在一系列醇中进行测量进行了校准,并使用曙红Y与甲基-β-环糊精的络合进行了测试。用Na +,K +-,H +,K +-和肌浆网Ca2 + -ATPase获得的结果表明,已知的曙红结合的ATP结合位点在Na +,K +-和H +的情况下明显更具极性,K + -ATPases与Ca2 + -ATPase相比。发现该结果与曙红与Na +,K + -ATP酶和Ca 2 + -ATP酶的E 2构象状态的对接计算一致。荧光实验表明,曙红与Na +的E1构象的结合力更强,K + -ATPase比E2构象要好,但是在Ca2 + -ATPase的情况下,荧光实验和对接计算都表明这两个构象之间的结合亲和力没有显着差异。此结果可能是由于以下事实:与Na +,K +-和H +,K + -ATPase相比,Ca2 + -ATPase的E2-E1转换不涉及富含赖氨酸的N末端尾巴的运动,可能会影响整体酶的构象。与此假设相符,N-端截短后,Na +,K + -ATPase E1构象的曙红亲和力显着降低。建议在E2-E1转换过程中Na +,K +-和H +,K + -ATPase的N末端尾部的构象熵的变化可能影响E1构象的热力学稳定性,从而影响其ATP结合亲和力。但是在Ca2 + -ATPase的情况下,荧光实验和对接计算都显示两种构象之间的结合亲和力没有显着差异。此结果可能是由于以下事实:与Na +,K +-和H +,K + -ATPase相比,Ca2 + -ATPase的E2-E1转换不涉及富含赖氨酸的N末端尾巴的运动,可能会影响整体酶的构象。与此假设相符,N-端截短后,Na +,K + -ATPase E1构象的曙红亲和力显着降低。建议在E2-E1转换过程中Na +,K +-和H +,K + -ATPase的N末端尾部的构象熵的变化可能影响E1构象的热力学稳定性,从而影响其ATP结合亲和力。但是在Ca2 + -ATPase的情况下,荧光实验和对接计算都显示两种构象之间的结合亲和力没有显着差异。此结果可能是由于以下事实:与Na +,K +-和H +,K + -ATPase相比,Ca2 + -ATPase的E2-E1转换不涉及富含赖氨酸的N末端尾巴的运动,可能会影响整体酶的构象。与此假设相符,N-端截短后,Na +,K + -ATPase E1构象的曙红亲和力显着降低。建议在E2-E1转换过程中Na +,K +-和H +,K + -ATPase的N末端尾部的构象熵的变化可能影响E1构象的热力学稳定性,从而影响其ATP结合亲和力。
更新日期:2019-11-30
中文翻译:

Na +,K + -ATPase,胃H +,K + -ATPase和肌浆网Ca2 + -ATPase的ATP结合位点的极性。
提出了一种利用探针曙红Y的荧光比率法,用于估计不同构象状态下P型ATP酶的ATP结合位点极性。该方法已通过在一系列醇中进行测量进行了校准,并使用曙红Y与甲基-β-环糊精的络合进行了测试。用Na +,K +-,H +,K +-和肌浆网Ca2 + -ATPase获得的结果表明,已知的曙红结合的ATP结合位点在Na +,K +-和H +的情况下明显更具极性,K + -ATPases与Ca2 + -ATPase相比。发现该结果与曙红与Na +,K + -ATP酶和Ca 2 + -ATP酶的E 2构象状态的对接计算一致。荧光实验表明,曙红与Na +的E1构象的结合力更强,K + -ATPase比E2构象要好,但是在Ca2 + -ATPase的情况下,荧光实验和对接计算都表明这两个构象之间的结合亲和力没有显着差异。此结果可能是由于以下事实:与Na +,K +-和H +,K + -ATPase相比,Ca2 + -ATPase的E2-E1转换不涉及富含赖氨酸的N末端尾巴的运动,可能会影响整体酶的构象。与此假设相符,N-端截短后,Na +,K + -ATPase E1构象的曙红亲和力显着降低。建议在E2-E1转换过程中Na +,K +-和H +,K + -ATPase的N末端尾部的构象熵的变化可能影响E1构象的热力学稳定性,从而影响其ATP结合亲和力。但是在Ca2 + -ATPase的情况下,荧光实验和对接计算都显示两种构象之间的结合亲和力没有显着差异。此结果可能是由于以下事实:与Na +,K +-和H +,K + -ATPase相比,Ca2 + -ATPase的E2-E1转换不涉及富含赖氨酸的N末端尾巴的运动,可能会影响整体酶的构象。与此假设相符,N-端截短后,Na +,K + -ATPase E1构象的曙红亲和力显着降低。建议在E2-E1转换过程中Na +,K +-和H +,K + -ATPase的N末端尾部的构象熵的变化可能影响E1构象的热力学稳定性,从而影响其ATP结合亲和力。但是在Ca2 + -ATPase的情况下,荧光实验和对接计算都显示两种构象之间的结合亲和力没有显着差异。此结果可能是由于以下事实:与Na +,K +-和H +,K + -ATPase相比,Ca2 + -ATPase的E2-E1转换不涉及富含赖氨酸的N末端尾巴的运动,可能会影响整体酶的构象。与此假设相符,N-端截短后,Na +,K + -ATPase E1构象的曙红亲和力显着降低。建议在E2-E1转换过程中Na +,K +-和H +,K + -ATPase的N末端尾部的构象熵的变化可能影响E1构象的热力学稳定性,从而影响其ATP结合亲和力。


























 京公网安备 11010802027423号
京公网安备 11010802027423号