当前位置:
X-MOL 学术
›
Bioorg. Med. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Design and synthesis of boron-containing diphenylpyrimidines as potent BTK and JAK3 dual inhibitors.
Bioorganic & Medicinal Chemistry ( IF 3.5 ) Pub Date : 2019-11-30 , DOI: 10.1016/j.bmc.2019.115236 Jing Ren 1 , Wei Shi 1 , Damin Zhao 1 , Qinglin Wang 1 , Xiayun Chang 1 , Xiangyi He 1 , Xiaojin Wang 1 , Yong Gao 1 , Peng Lu 1 , Xiquan Zhang 1 , Hongjiang Xu 1 , Yinsheng Zhang 1
Bioorganic & Medicinal Chemistry ( IF 3.5 ) Pub Date : 2019-11-30 , DOI: 10.1016/j.bmc.2019.115236 Jing Ren 1 , Wei Shi 1 , Damin Zhao 1 , Qinglin Wang 1 , Xiayun Chang 1 , Xiangyi He 1 , Xiaojin Wang 1 , Yong Gao 1 , Peng Lu 1 , Xiquan Zhang 1 , Hongjiang Xu 1 , Yinsheng Zhang 1
Affiliation
|
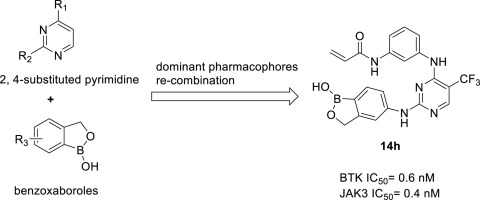
Bruton's tyrosine kinase (BTK) and Janus kinase 3 (JAK3) are very promising targets for hematological malignancies and autoimmune diseases. In recent years, a few compounds have been approved as a marketed medicine, and several are undergoing clinical trials. By recombining the dominant backbone of known active compounds, constructing a foused library, and screening a broad panel of kinases, we found a class of compounds with dual activities of anti-BTK and anti-JAK3. Some of the compounds have shown 10-folds more active in the enzyme and cell-based assays than a known active compound. Furthermore, liver microsome stability experiments show that these compounds have better stability than ibrutinib. These explorations offered new clues to discover benzoxaborole fragment and pyrimidine scaffold as more effective BTK and JAK3 dual inhibitors.
中文翻译:

设计和合成含硼二苯基嘧啶作为有效的BTK和JAK3双重抑制剂。
Bruton的酪氨酸激酶(BTK)和Janus激酶3(JAK3)是血液恶性肿瘤和自身免疫性疾病非常有希望的靶标。近年来,一些化合物已被批准作为市售药物,并且一些正在接受临床试验。通过重组已知活性化合物的主要骨架,构建数据库和筛选广泛的激酶,我们发现了一类具有抗BTK和抗JAK3双重活性的化合物。在酶和基于细胞的测定中,某些化合物的活性已显示出比已知活性化合物高10倍。此外,肝微粒体稳定性实验表明,这些化合物具有比依鲁替尼更好的稳定性。这些探索提供了新的线索,以发现作为更有效的BTK和JAK3双重抑制剂的苯并x硼烷片段和嘧啶支架。
更新日期:2019-11-30
中文翻译:

设计和合成含硼二苯基嘧啶作为有效的BTK和JAK3双重抑制剂。
Bruton的酪氨酸激酶(BTK)和Janus激酶3(JAK3)是血液恶性肿瘤和自身免疫性疾病非常有希望的靶标。近年来,一些化合物已被批准作为市售药物,并且一些正在接受临床试验。通过重组已知活性化合物的主要骨架,构建数据库和筛选广泛的激酶,我们发现了一类具有抗BTK和抗JAK3双重活性的化合物。在酶和基于细胞的测定中,某些化合物的活性已显示出比已知活性化合物高10倍。此外,肝微粒体稳定性实验表明,这些化合物具有比依鲁替尼更好的稳定性。这些探索提供了新的线索,以发现作为更有效的BTK和JAK3双重抑制剂的苯并x硼烷片段和嘧啶支架。


























 京公网安备 11010802027423号
京公网安备 11010802027423号