当前位置:
X-MOL 学术
›
J. Chin. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Comments on chemical properties reported for diphenyl disulfide and its derivatives: The merit of the phenyl groups
Journal of the Chinese Chemical Society ( IF 1.8 ) Pub Date : 2019-11-28 , DOI: 10.1002/jccs.201900317 Hung‐Sung Lin, Yan Wu, Yu‐Ju Liu, Shu‐Hui Chen, Wei‐Ting Chen, Shao‐Pin Wang
Journal of the Chinese Chemical Society ( IF 1.8 ) Pub Date : 2019-11-28 , DOI: 10.1002/jccs.201900317 Hung‐Sung Lin, Yan Wu, Yu‐Ju Liu, Shu‐Hui Chen, Wei‐Ting Chen, Shao‐Pin Wang
|
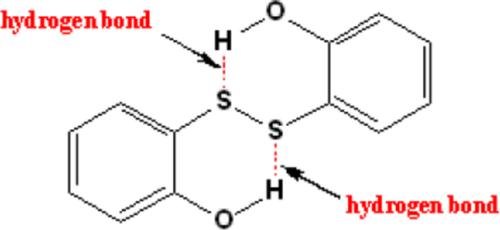
The symmetrical 2,2′‐disubstitued derivatives of diphenyl disulfide showing widely spanning rates of electrophilic attack of the HIV‐1 nucleocapsid protein p7 zinc fingers have been rationalized, based on the lowest unoccupied molecular orbital (LUMO)‐lowering approach, by the substituents' π‐effects and the hydrogen bond stabilization effects. In the 2,2′‐amide‐ and 4,4′‐N‐amide‐substituted derivatives, the extent of LUMO lowering has been reduced by the destabilization of lone‐pair bond orbital, lp(N), present on the nitrogen atom of N‐amide. From the natural bond orbital viewpoint, hydrogen bond stabilization of LUMO is mainly governed by stabilization of the σ*SS bond orbital.
中文翻译:

关于二苯基二硫化物及其衍生物的化学性质的评论:苯基的优点
根据最低的未占用分子轨道(LUMO)降低方法,已通过取代基合理化了对称的2,2'-二硫化二苯取代衍生物,显示了HIV-1核衣壳蛋白p7锌指的广泛的亲电子攻击速率π效应和氢键稳定效应。在2,2'-酰胺和4,4'-N-酰胺取代的衍生物中,氮原子上存在的孤对键轨道lp(N)失稳,降低了LUMO降低的程度N-酰胺。从自然键轨道的角度来看,LUMO的氢键稳定主要由σ* SS键轨道的稳定决定。
更新日期:2020-02-14
中文翻译:

关于二苯基二硫化物及其衍生物的化学性质的评论:苯基的优点
根据最低的未占用分子轨道(LUMO)降低方法,已通过取代基合理化了对称的2,2'-二硫化二苯取代衍生物,显示了HIV-1核衣壳蛋白p7锌指的广泛的亲电子攻击速率π效应和氢键稳定效应。在2,2'-酰胺和4,4'-N-酰胺取代的衍生物中,氮原子上存在的孤对键轨道lp(N)失稳,降低了LUMO降低的程度N-酰胺。从自然键轨道的角度来看,LUMO的氢键稳定主要由σ* SS键轨道的稳定决定。


























 京公网安备 11010802027423号
京公网安备 11010802027423号