Journal of Organometallic Chemistry ( IF 2.3 ) Pub Date : 2019-11-29 , DOI: 10.1016/j.jorganchem.2019.121056 Anas Lataifeh
|
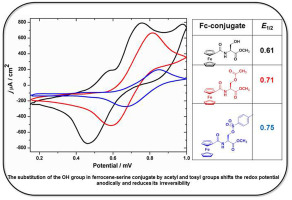
Mono- and disubstituted ferrocenoyl amino acid conjugates having free hydroxyl (OH) group at the amino acid side chain is synthesized, namely Fc–CO–aa-OCH3 (1a, 2a, 3a), and Fc-[CO-aa-OCH3]2 (1b, 2b, 3b), Fc = ferrocene, aa = l-serine (L-Ser, 1), l-tyrosine (L-Tyr, 2), l-threonine (L-Thr, 3). The reactivity of the OH group in 1a toward substitution reaction by acetyl chloride, p-toluene sulfonyl chloride and phosphoric acid is investigated. The resulting compounds are Fc–CO–L-Ser(C(O)–CH3)–OCH3 (1c), Fc–CO–L-Ser(S(O)2–C6H4–CH3)–OCH3 (1d) and Fc–CO–L-Ser(P(O)–(OH)2)–OCH3 (1e). The prepared Fc-amino acid conjugates are fully characterized by standard spectroscopic methods. The cyclic voltammetry of the Fc-compounds show a quasi-reversibility for conjugates 1a-3a (E1/2 = 0.64 V) and for 1b, 3b (E1/2 = 0.85 V), while an irreversible behavior for 2b is observed. The compounds 1c and 1d exhibit quasi-reversibility with E1/2 = 0.71 V, which is shifted anodically by 100 mV compared to the parent conjugate 1a. Fc-conjugate 1e shows complete irreversibility. The study suggests that profound changes in Fc-redox potential is accessible through varying the substituent at the OH group in the amino acid side chain, either by anodic shift of the Fc signal (acylation and tosylation) or turn the signal off by phosphorylation.
中文翻译:

含羟基的侧链氨基酸的二茂铁基共轭物:合成,电化学研究和对亲电试剂的反应性
合成了在氨基酸侧链具有游离羟基(OH)的单取代和二取代的二茂铁酰基氨基酸共轭物,即Fc-CO-aa-OCH 3(1a,2a,3a)和Fc- [CO-aa-OCH 3 ] 2(1b,2b,3b),Fc =二茂铁,aa = 1-丝氨酸(L - Ser ,1),1-酪氨酸(L - Tyr ,2),1-苏氨酸(L- Thr,3)。1a中OH基团的反应性对乙酰氯,对甲苯磺酰氯和磷酸的取代反应进行了研究。生成的化合物为Fc-CO- L - Ser(C(O)-CH 3)-OCH 3(1c),Fc-CO- L - Ser(S(O)2 -C 6 H 4 -CH 3)- OCH 3(1d)和Fc–CO– L -Ser(P(O)–(OH)2)–OCH 3(1e)。所制备的Fc-氨基酸缀合物通过标准光谱法充分表征。Fc化合物的循环伏安法显示了结合物1a - 3a(E 1/2 = 0.64 V)和1b,3b(E 1/2 = 0.85 V)的准可逆性,而观察到2b的不可逆行为。化合物1c和1d表现出准可逆性,E 1/2 = 0.71 V,与母体缀合物1a相比,其阳极移位100 mV 。Fc缀合物1e显示完全不可逆转。这项研究表明,通过改变氨基酸信号侧链(酰化和甲苯磺酸化)或通过磷酸化关闭信号,可以通过改变氨基酸侧链的OH基团上的取代基来获得Fc-氧化还原电位的深远变化。


























 京公网安备 11010802027423号
京公网安备 11010802027423号