当前位置:
X-MOL 学术
›
Metallomics
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Copper nanoparticles induce zebrafish intestinal defects via endoplasmic reticulum and oxidative stress.
Metallomics ( IF 3.4 ) Pub Date : 2020-01-29 , DOI: 10.1039/c9mt00210c Guang Zhao 1 , Ting Zhang , HaoJie Sun , Jing-Xia Liu
Metallomics ( IF 3.4 ) Pub Date : 2020-01-29 , DOI: 10.1039/c9mt00210c Guang Zhao 1 , Ting Zhang , HaoJie Sun , Jing-Xia Liu
Affiliation
|
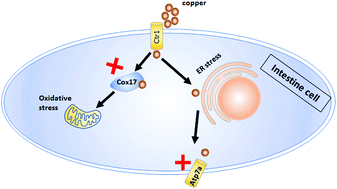
As an essential trace element, copper plays key roles in the activation of multiple enzymes, neurotransmitter biosynthesis and denaturation, as well as the decomposition of superoxide and the synthesis of collagen. The intestines is the main organ for copper absorption and transfer, and intestinal copper accumulation is observed in some patients with gene mutations. However, a vertebrate model to link copper accumulation with intestinal diseases and defects is still lacking, and the data concerning the mechanisms underlying this link are still scarce. In this study, the effects of exogenous copper (CuNPs or their released Cu2+) on intestinal development in zebrafish embryos were investigated after their exposure to copper. The results showed that over 0.10 mg L-1 of CuNPs or Cu2+ damaged the zebrafish intestinal development, including thinned epithelial cells as well as few and shortened intestinal villi. Under CuNP stress, the expression was significantly (p < 0.05) reduced for the intestinal marker genes (slc15a1b, cyp3a65, cyp8b1, fabp2), but increased for the endoplasmic reticulum (ER) stress marker (bip) in the zebrafish intestines. Additionally, immunofluorescence analysis revealed that CuNPs or Cu2+ induced the production of ER stress (indicated by PDI) and oxidative stress (indicated by 4-HNE) in the intestinal cells. The expression of the aforementioned intestinal marker genes could be restored to normal by inhibiting the production of ER stress or oxidative stress with ER stress alleviator PBA (4-phenylbutyric acid) or ROS scavengers GSH (reduced Glutathione) or NAC (Nacetylcysteine) in CuNP or Cu2+ stressed embryos, suggesting that copper induces intestinal defects mainly by ER and oxidative stress. Moreover, obvious intestinal defects were observed in copper-stressed cox17-/- and atp7a-/- mutants, implying that blocking the transportation of copper to the mitochondria or trans-Golgi network by deleting cox17 or atp7a could not alleviate copper-induced intestinal developmental defects. This is probably the first report to reveal that copper nanoparticles (CuNPs) and their released ions (Cu2+) cause intestinal developmental defects via inducing ER and ROS stresses. It is also the first report on the intestinal developmental responses of cox17-/- or atp7a-/- mutants to copper stimulation.
中文翻译:

铜纳米颗粒通过内质网和氧化应激诱导斑马鱼肠道缺陷。
作为必需的微量元素,铜在多种酶的活化,神经递质的生物合成和变性以及超氧化物的分解和胶原蛋白的合成中起着关键作用。肠是铜吸收和转移的主要器官,并且在一些具有基因突变的患者中观察到肠中铜的积累。但是,仍然缺乏将铜积累与肠道疾病和缺陷联系起来的脊椎动物模型,关于这种联系背后的机理的数据仍然很少。在这项研究中,研究了外源铜(CuNPs或它们释放的Cu2 +)对斑马鱼胚胎暴露于铜后对肠道发育的影响。结果表明,超过0.10 mg L-1的CuNPs或Cu2 +破坏了斑马鱼的肠道发育,包括上皮细胞变薄,以及肠绒毛少而又短的绒毛。在CuNP胁迫下,肠标记基因(slc15a1b,cyp3a65,cyp8b1,fabp2)的表达显着降低(p <0.05),但在斑马鱼肠中的内质网(ER)胁迫标记(bip)表达升高。此外,免疫荧光分析表明,CuNPs或Cu2 +诱导了肠细胞中ER应激(由PDI指示)和氧化应激(由4-HNE指示)的产生。可以通过用CuNP或PNP中的ER应激缓解剂PBA(4-苯基丁酸)或ROS清除剂GSH(还原型谷胱甘肽)或NAC(N乙酰基半胱氨酸)抑制ER应激或氧化应激的产生来恢复上述肠道标记基因的表达。 Cu2 +胁迫的胚胎,提示铜主要通过内质网和氧化应激诱导肠道缺陷。此外,在铜胁迫的cox17-/-和atp7a-/-突变体中观察到明显的肠道缺陷,这意味着通过删除cox17或atp7a阻止铜向线粒体或反高尔基体网络的运输不能减轻铜诱导的肠道发育。缺陷。这可能是第一个揭示铜纳米颗粒(CuNPs)及其释放的离子(Cu2 +)通过诱导ER和ROS应力导致肠道发育缺陷的报告。这也是关于cox17-/-或atp7a-/-突变体对铜刺激的肠道发育反应的第一份报告。暗示通过删除cox17或atp7a阻止铜向线粒体或反高尔基体网络的运输不能减轻铜诱导的肠发育缺陷。这可能是第一个揭示铜纳米颗粒(CuNPs)及其释放的离子(Cu2 +)通过诱导ER和ROS应力引起肠道发育缺陷的报告。这也是关于cox17-/-或atp7a-/-突变体对铜刺激的肠道发育反应的第一份报告。暗示通过删除cox17或atp7a阻止铜向线粒体或反高尔基体网络的运输不能减轻铜诱导的肠发育缺陷。这可能是第一个揭示铜纳米颗粒(CuNPs)及其释放的离子(Cu2 +)通过诱导ER和ROS应力导致肠道发育缺陷的报告。这也是关于cox17-/-或atp7a-/-突变体对铜刺激的肠道发育反应的第一份报告。
更新日期:2020-02-14
中文翻译:

铜纳米颗粒通过内质网和氧化应激诱导斑马鱼肠道缺陷。
作为必需的微量元素,铜在多种酶的活化,神经递质的生物合成和变性以及超氧化物的分解和胶原蛋白的合成中起着关键作用。肠是铜吸收和转移的主要器官,并且在一些具有基因突变的患者中观察到肠中铜的积累。但是,仍然缺乏将铜积累与肠道疾病和缺陷联系起来的脊椎动物模型,关于这种联系背后的机理的数据仍然很少。在这项研究中,研究了外源铜(CuNPs或它们释放的Cu2 +)对斑马鱼胚胎暴露于铜后对肠道发育的影响。结果表明,超过0.10 mg L-1的CuNPs或Cu2 +破坏了斑马鱼的肠道发育,包括上皮细胞变薄,以及肠绒毛少而又短的绒毛。在CuNP胁迫下,肠标记基因(slc15a1b,cyp3a65,cyp8b1,fabp2)的表达显着降低(p <0.05),但在斑马鱼肠中的内质网(ER)胁迫标记(bip)表达升高。此外,免疫荧光分析表明,CuNPs或Cu2 +诱导了肠细胞中ER应激(由PDI指示)和氧化应激(由4-HNE指示)的产生。可以通过用CuNP或PNP中的ER应激缓解剂PBA(4-苯基丁酸)或ROS清除剂GSH(还原型谷胱甘肽)或NAC(N乙酰基半胱氨酸)抑制ER应激或氧化应激的产生来恢复上述肠道标记基因的表达。 Cu2 +胁迫的胚胎,提示铜主要通过内质网和氧化应激诱导肠道缺陷。此外,在铜胁迫的cox17-/-和atp7a-/-突变体中观察到明显的肠道缺陷,这意味着通过删除cox17或atp7a阻止铜向线粒体或反高尔基体网络的运输不能减轻铜诱导的肠道发育。缺陷。这可能是第一个揭示铜纳米颗粒(CuNPs)及其释放的离子(Cu2 +)通过诱导ER和ROS应力导致肠道发育缺陷的报告。这也是关于cox17-/-或atp7a-/-突变体对铜刺激的肠道发育反应的第一份报告。暗示通过删除cox17或atp7a阻止铜向线粒体或反高尔基体网络的运输不能减轻铜诱导的肠发育缺陷。这可能是第一个揭示铜纳米颗粒(CuNPs)及其释放的离子(Cu2 +)通过诱导ER和ROS应力引起肠道发育缺陷的报告。这也是关于cox17-/-或atp7a-/-突变体对铜刺激的肠道发育反应的第一份报告。暗示通过删除cox17或atp7a阻止铜向线粒体或反高尔基体网络的运输不能减轻铜诱导的肠发育缺陷。这可能是第一个揭示铜纳米颗粒(CuNPs)及其释放的离子(Cu2 +)通过诱导ER和ROS应力导致肠道发育缺陷的报告。这也是关于cox17-/-或atp7a-/-突变体对铜刺激的肠道发育反应的第一份报告。


























 京公网安备 11010802027423号
京公网安备 11010802027423号