当前位置:
X-MOL 学术
›
Hydrometallurgy
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Interaction mechanism between marmatite and chalcocite in acidic (microbial) environments
Hydrometallurgy ( IF 4.7 ) Pub Date : 2020-01-01 , DOI: 10.1016/j.hydromet.2019.105217 Yisheng Zhang , Hongbo Zhao , Yanjun Zhang , Hongwei Liu , Huaqun Yin , Jiushuai Deng , Guanzhou Qiu
Hydrometallurgy ( IF 4.7 ) Pub Date : 2020-01-01 , DOI: 10.1016/j.hydromet.2019.105217 Yisheng Zhang , Hongbo Zhao , Yanjun Zhang , Hongwei Liu , Huaqun Yin , Jiushuai Deng , Guanzhou Qiu
|
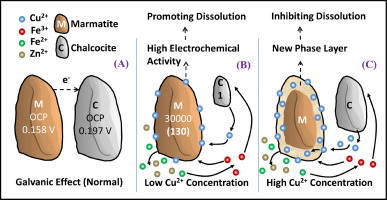
Abstract Sulphide minerals commonly existing in the form of polymetallic ores are the essential objects in the fields of acid mine drainage and biohydrometallurgy. Although interactions between different minerals have been studied extensively, there are no research reports on the interactions between marmatite and chalcocite. Hence, the interactions between marmatite and chalcocite in biotic/abiotic leaching and electrochemical systems were investigated. Electrochemical tests show that the open circuit potential of marmatite is 39 mV lower than that of chalcocite. Theoretically, marmatite should react as an anode in this galvanic couple, thus dissolving faster. However, leaching results suggest that the introduction of marmatite promotes chalcocite dissolution but its own dissolution was impeded, despite a slight acceleration observed during the early stage in abiotic systems. The results of this study reveal that the solution factors including oxidation-reduction potential, iron concentrations, and microbial concentrations might have significant effects that are more potent than the galvanic effect. The unpredictable inhibition of marmatite dissolution might be attributable to the existence of excess Cu2+ liberated from the chalcocite. A further deduction proposed that a synergistic dissolution of marmatite and chalcocite can be achieved by adjusting their mass ratios to attain a suitable Cu2+ concentration.
中文翻译:

酸性(微生物)环境中铁铜矿和辉铜矿之间的相互作用机制
摘要 硫化物矿物通常以多金属矿的形式存在,是酸性矿山排水和生物湿法冶金领域的重要对象。虽然对不同矿物之间的相互作用进行了广泛的研究,但目前还没有关于铁闪石和辉铜矿之间相互作用的研究报告。因此,研究了生物/非生物浸出和电化学系统中铁铜矿和辉铜矿之间的相互作用。电化学测试表明,铁闪石的开路电位比辉铜矿低39 mV。从理论上讲,铁闪石应该作为该电偶中的阳极反应,从而更快地溶解。然而,浸出结果表明,铁闪石的引入促进了辉铜矿的溶解,但其自身的溶解受到了阻碍,尽管在非生物系统的早期阶段观察到了轻微的加速。这项研究的结果表明,包括氧化还原电位、铁浓度和微生物浓度在内的溶液因素可能具有比电流效应更有效的显着影响。铁闪石溶解的不可预测的抑制可能归因于从辉铜矿中释放出的过量 Cu2+ 的存在。进一步推论提出,通过调整它们的质量比以获得合适的 Cu2+ 浓度,可以实现铁铜矿和辉铜矿的协同溶解。微生物浓度可能具有比电流效应更有效的显着影响。铁闪石溶解的不可预测的抑制可能归因于从辉铜矿中释放出的过量 Cu2+ 的存在。进一步推论提出,通过调整它们的质量比以获得合适的 Cu2+ 浓度,可以实现铁铜矿和辉铜矿的协同溶解。微生物浓度可能具有比电流效应更有效的显着影响。铁闪石溶解的不可预测的抑制可能归因于从辉铜矿中释放出的过量 Cu2+ 的存在。进一步推论提出,通过调整它们的质量比以获得合适的 Cu2+ 浓度,可以实现铁铜矿和辉铜矿的协同溶解。
更新日期:2020-01-01
中文翻译:

酸性(微生物)环境中铁铜矿和辉铜矿之间的相互作用机制
摘要 硫化物矿物通常以多金属矿的形式存在,是酸性矿山排水和生物湿法冶金领域的重要对象。虽然对不同矿物之间的相互作用进行了广泛的研究,但目前还没有关于铁闪石和辉铜矿之间相互作用的研究报告。因此,研究了生物/非生物浸出和电化学系统中铁铜矿和辉铜矿之间的相互作用。电化学测试表明,铁闪石的开路电位比辉铜矿低39 mV。从理论上讲,铁闪石应该作为该电偶中的阳极反应,从而更快地溶解。然而,浸出结果表明,铁闪石的引入促进了辉铜矿的溶解,但其自身的溶解受到了阻碍,尽管在非生物系统的早期阶段观察到了轻微的加速。这项研究的结果表明,包括氧化还原电位、铁浓度和微生物浓度在内的溶液因素可能具有比电流效应更有效的显着影响。铁闪石溶解的不可预测的抑制可能归因于从辉铜矿中释放出的过量 Cu2+ 的存在。进一步推论提出,通过调整它们的质量比以获得合适的 Cu2+ 浓度,可以实现铁铜矿和辉铜矿的协同溶解。微生物浓度可能具有比电流效应更有效的显着影响。铁闪石溶解的不可预测的抑制可能归因于从辉铜矿中释放出的过量 Cu2+ 的存在。进一步推论提出,通过调整它们的质量比以获得合适的 Cu2+ 浓度,可以实现铁铜矿和辉铜矿的协同溶解。微生物浓度可能具有比电流效应更有效的显着影响。铁闪石溶解的不可预测的抑制可能归因于从辉铜矿中释放出的过量 Cu2+ 的存在。进一步推论提出,通过调整它们的质量比以获得合适的 Cu2+ 浓度,可以实现铁铜矿和辉铜矿的协同溶解。


























 京公网安备 11010802027423号
京公网安备 11010802027423号