Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Matrix-assisted nanoelectrospray mass spectrometry for soft ionization of metal(i)-protein complexes.
Analyst ( IF 4.2 ) Pub Date : 2019-12-20 , DOI: 10.1039/c9an02117e Jin Li 1 , Yajun Zheng 1 , Jia Zhao 1 , Daniel E Austin 2 , Zhiping Zhang 1
Analyst ( IF 4.2 ) Pub Date : 2019-12-20 , DOI: 10.1039/c9an02117e Jin Li 1 , Yajun Zheng 1 , Jia Zhao 1 , Daniel E Austin 2 , Zhiping Zhang 1
Affiliation
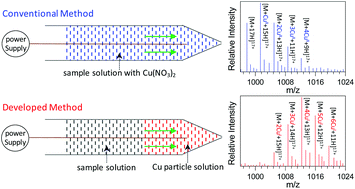
|
Metal ions play significant roles in biological processes, and investigation of metal-protein interactions provides a basis to understand the functions of metal ions in such systems. In the current study, a novel matrix-assisted nanoelectrospray ionization mass spectrometry (MANESI-MS) method was developed for investigating the interactions between metal ions (i.e., Cu+) and protein molecules (i.e., myoglobin) using Cu nanoparticles as the matrix. The results demonstrated that the present method not only was an efficient strategy for the generation of various complexes with monovalent metal ions, such as Cu+, in which no redox transitions between Cu+ and Cu2+ were observed, but also allowed a softer ionization of the generated Cu+-myoglobin complexes compared to that of myoglobin molecules with conventional nanoESI. Several parameters (i.e., the mixing mode of the myoglobin sample and Cu nanoparticle solution, size of the Cu particle, oxidation state of the Cu species, and acidity of the myoglobin solution) were found to be crucial in determining the ionization efficiency of the MANESI method. First loading a Cu nanoparticle solution into the electrospray tip followed by a myoglobin solution resulted in a favorable interaction between the generated Cu+ ions and myoglobin molecules, in which a smaller size of the Cu particle and a lower oxidation state of the metal species (Cu > Cu2O > CuO) gave a lower average charge state and hence a softer ionization of the resulting Cu+-myoglobin complexes, possibly due to the reduced denaturing effects of the Cu+ complex. The MANESI method has also been successfully used to ionize the complexes between Cu+ and other biological molecules such as cytochrome c and angiotension II, although an exception was found for lysozymes, which show an increase in the charge state. Analogous to the study with Cu, a variety of other metal nanoparticles (Ni, Fe, W, Ag, Al, Zn and Co) were explored to study their interactions with myoglobin, but only Zn and Co could produce monovalent metal ions (i.e., Zn+ and Co+) followed by a favorable interaction with myoglobin, and a soft ionization of the resulting complexes.
中文翻译:

基质辅助纳米电喷雾质谱用于金属(i)-蛋白质复合物的软电离。
金属离子在生物过程中起重要作用,对金属-蛋白质相互作用的研究为了解金属离子在此类系统中的功能提供了基础。在当前的研究中,开发了一种新颖的基质辅助纳米电喷雾电离质谱(MANESI-MS)方法,用于研究以铜纳米颗粒为基质的金属离子(即Cu +)和蛋白质分子(即肌红蛋白)之间的相互作用。结果表明,本方法不仅是一种有效的策略,用于生成各种与单价金属离子(例如Cu +)形成的络合物,其中未观察到Cu +和Cu2 +之间的氧化还原转变,而且还使生成的Cu +的电离更软。 -肌红蛋白复合物与使用常规nanoESI的肌红蛋白分子相比。几个参数(即,发现肌红蛋白样品和Cu纳米颗粒溶液的混合模式,Cu颗粒的大小,Cu物种的氧化态和肌红蛋白溶液的酸度对于确定MANESI方法的电离效率至关重要。首先将Cu纳米颗粒溶液加载到电喷雾头中,然后将肌红蛋白溶液加载到所产生的Cu +离子和肌红蛋白分子之间,从而产生有利的相互作用,其中较小的Cu颗粒尺寸和较低的金属物种氧化态(Cu> Cu 2 O> CuO)的平均电荷态较低,因此所得的Cu +-肌红蛋白络合物的电离较软,这可能是由于Cu +络合物的变性作用降低所致。MANESI方法也已成功用于电离Cu +与其他生物分子(例如细胞色素c和血管紧张素II)之间的复合物,尽管发现溶菌酶例外,其显示电荷状态增加。类似于对Cu的研究,还探索了多种其他金属纳米粒子(Ni,Fe,W,Ag,Al,Zn和Co)以研究它们与肌红蛋白的相互作用,但只有Zn和Co可以产生单价金属离子(即, Zn +和Co +),然后与肌红蛋白发生良好的相互作用,并使生成的络合物发生软化。
更新日期:2020-03-03
中文翻译:

基质辅助纳米电喷雾质谱用于金属(i)-蛋白质复合物的软电离。
金属离子在生物过程中起重要作用,对金属-蛋白质相互作用的研究为了解金属离子在此类系统中的功能提供了基础。在当前的研究中,开发了一种新颖的基质辅助纳米电喷雾电离质谱(MANESI-MS)方法,用于研究以铜纳米颗粒为基质的金属离子(即Cu +)和蛋白质分子(即肌红蛋白)之间的相互作用。结果表明,本方法不仅是一种有效的策略,用于生成各种与单价金属离子(例如Cu +)形成的络合物,其中未观察到Cu +和Cu2 +之间的氧化还原转变,而且还使生成的Cu +的电离更软。 -肌红蛋白复合物与使用常规nanoESI的肌红蛋白分子相比。几个参数(即,发现肌红蛋白样品和Cu纳米颗粒溶液的混合模式,Cu颗粒的大小,Cu物种的氧化态和肌红蛋白溶液的酸度对于确定MANESI方法的电离效率至关重要。首先将Cu纳米颗粒溶液加载到电喷雾头中,然后将肌红蛋白溶液加载到所产生的Cu +离子和肌红蛋白分子之间,从而产生有利的相互作用,其中较小的Cu颗粒尺寸和较低的金属物种氧化态(Cu> Cu 2 O> CuO)的平均电荷态较低,因此所得的Cu +-肌红蛋白络合物的电离较软,这可能是由于Cu +络合物的变性作用降低所致。MANESI方法也已成功用于电离Cu +与其他生物分子(例如细胞色素c和血管紧张素II)之间的复合物,尽管发现溶菌酶例外,其显示电荷状态增加。类似于对Cu的研究,还探索了多种其他金属纳米粒子(Ni,Fe,W,Ag,Al,Zn和Co)以研究它们与肌红蛋白的相互作用,但只有Zn和Co可以产生单价金属离子(即, Zn +和Co +),然后与肌红蛋白发生良好的相互作用,并使生成的络合物发生软化。


























 京公网安备 11010802027423号
京公网安备 11010802027423号