Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
CRL3-SPOP ubiquitin ligase complex suppresses the growth of diffuse large B-cell lymphoma by negatively regulating the MyD88/NF-κB signaling.
Leukemia ( IF 11.4 ) Pub Date : 2019-11-27 , DOI: 10.1038/s41375-019-0661-z Xiaofeng Jin 1 , Qing Shi 2 , Qian Li 1 , Linyi Zhou 3 , Jian Wang 1 , Lei Jiang 1 , Xiaying Zhao 2 , Kai Feng 2 , Ting Lin 1 , Zihan Lin 1 , Hui Zhuang 1 , Jianye Yang 1 , Chongke Hu 1 , Luyi Zhang 1 , Liliang Shen 4 , Ying Lu 4 , Jie Zhu 5 , Haibiao Wang 5 , Honggang Qi 6 , Xiaodan Meng 1 , Yang Xi 1 , Jinchang Pan 1 , Shuai Fang 1 , Haihua Tian 1 , Chengwei Zhou 7 , Pingzhao Zhang 8 , Kun Gao 9 , Shi-Min Zhao 2 , Yao Li 2 , Zhaohui Gong 1 , Chenji Wang 2
Leukemia ( IF 11.4 ) Pub Date : 2019-11-27 , DOI: 10.1038/s41375-019-0661-z Xiaofeng Jin 1 , Qing Shi 2 , Qian Li 1 , Linyi Zhou 3 , Jian Wang 1 , Lei Jiang 1 , Xiaying Zhao 2 , Kai Feng 2 , Ting Lin 1 , Zihan Lin 1 , Hui Zhuang 1 , Jianye Yang 1 , Chongke Hu 1 , Luyi Zhang 1 , Liliang Shen 4 , Ying Lu 4 , Jie Zhu 5 , Haibiao Wang 5 , Honggang Qi 6 , Xiaodan Meng 1 , Yang Xi 1 , Jinchang Pan 1 , Shuai Fang 1 , Haihua Tian 1 , Chengwei Zhou 7 , Pingzhao Zhang 8 , Kun Gao 9 , Shi-Min Zhao 2 , Yao Li 2 , Zhaohui Gong 1 , Chenji Wang 2
Affiliation
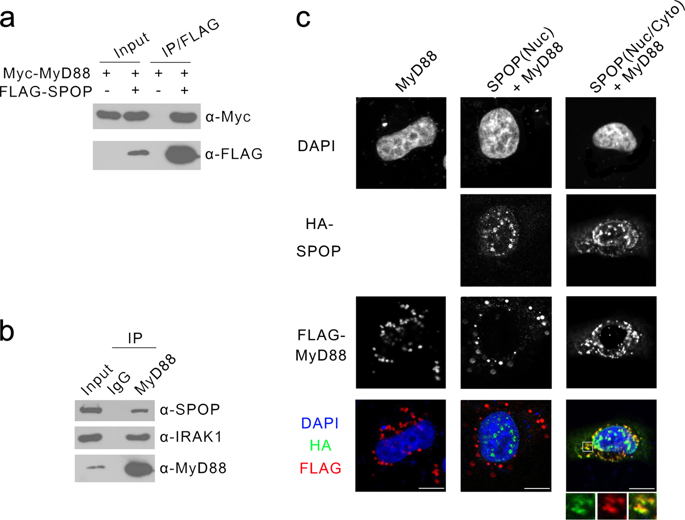
|
Recurrent oncogenic mutations of MyD88 have been identified in a variety of lymphoid malignancies. Gain-of-function mutations of MyD88 constitutively activate downstream NF-κB signaling pathways, resulting in increased cellular proliferation and survival. However, whether MyD88 activity can be aberrantly regulated in MyD88-wild-type lymphoid malignancies remains poorly understood. SPOP is an adaptor protein of CUL3-based E3 ubiquitin ligase complex and frequently mutated genes in prostate and endometrial cancers. In this study, we reveal that SPOP binds to and induces the nondegradative ubiquitination of MyD88 by recognizing an atypical SPOP-binding motif in MyD88. This modification blocks Myddosome assembly and downstream NF-κB activation. SPOP is mutated in a subset of lymphoid malignancies, including diffuse large B-cell lymphoma (DLBCL). Lymphoid malignancies-associated SPOP mutants exhibited impaired binding to MyD88 and suppression of NF-κB activation. The DLBCL-associated, SPOP-binding defective mutants of MyD88 escaped from SPOP-mediated ubiquitination, and their effect on NF-κB activation is stronger than that of wild-type MyD88. Moreover, SPOP suppresses DLBCL cell growth in vitro and tumor xenograft in vivo by inhibiting the MyD88/NF-κB signaling. Therefore, SPOP acts as a tumor suppressor in DLBCL. Mutations in the SPOP-MyD88 binding interface may disrupt the SPOP-MyD88 regulatory axis and promote aberrant MyD88/NF-κB activation and cell growth in DLCBL.
中文翻译:

CRL3-SPOP泛素连接酶复合物通过负调控MyD88 /NF-κB信号传导来抑制弥漫性大B细胞淋巴瘤的生长。
MyD88的复发性致癌突变已在多种淋巴恶性肿瘤中得到鉴定。MyD88的功能获得性突变组成性激活下游NF-κB信号通路,从而导致细胞增殖和存活增加。但是,MyD88活性是否可以在MyD88野生型淋巴恶性肿瘤中被异常调节仍然知之甚少。SPOP是基于CUL3的E3泛素连接酶复合物的衔接蛋白,经常在前列腺癌和子宫内膜癌中突变。在这项研究中,我们揭示了SPOP通过识别MyD88中的非典型SPOP结合基序与MyD88结合并诱导其非降解性泛素化。此修饰可阻止Myddosome装配和下游NF-κB激活。SPOP在淋巴恶性肿瘤的子集中发生突变,包括弥漫性大B细胞淋巴瘤(DLBCL)。淋巴恶性肿瘤相关的SPOP突变体与MyD88的结合受损,并抑制NF-κB活化。与DLBCL相关的,结合SPOP的MyD88缺陷突变体摆脱了SPOP介导的泛素化作用,其对NF-κB活化的作用强于野生型MyD88。此外,SPOP通过抑制MyD88 /NF-κB信号传导抑制体外DLBCL细胞生长和体内肿瘤异种移植。因此,SPOP在DLBCL中起着抑癌作用。SPOP-MyD88结合界面中的突变可能会破坏SPOP-MyD88调控轴并促进DLCBL中异常的MyD88 /NF-κB活化和细胞生长。而且它们对NF-κB活化的作用要强于野生型MyD88。此外,SPOP通过抑制MyD88 /NF-κB信号传导抑制体外DLBCL细胞生长和体内肿瘤异种移植。因此,SPOP在DLBCL中起着抑癌作用。SPOP-MyD88结合界面中的突变可能会破坏SPOP-MyD88调控轴并促进DLCBL中异常的MyD88 /NF-κB活化和细胞生长。而且它们对NF-κB活化的作用要强于野生型MyD88。此外,SPOP通过抑制MyD88 /NF-κB信号传导抑制体外DLBCL细胞生长和体内肿瘤异种移植。因此,SPOP在DLBCL中起着抑癌作用。SPOP-MyD88结合界面中的突变可能会破坏SPOP-MyD88调控轴并促进DLCBL中异常的MyD88 /NF-κB活化和细胞生长。
更新日期:2019-11-28
中文翻译:

CRL3-SPOP泛素连接酶复合物通过负调控MyD88 /NF-κB信号传导来抑制弥漫性大B细胞淋巴瘤的生长。
MyD88的复发性致癌突变已在多种淋巴恶性肿瘤中得到鉴定。MyD88的功能获得性突变组成性激活下游NF-κB信号通路,从而导致细胞增殖和存活增加。但是,MyD88活性是否可以在MyD88野生型淋巴恶性肿瘤中被异常调节仍然知之甚少。SPOP是基于CUL3的E3泛素连接酶复合物的衔接蛋白,经常在前列腺癌和子宫内膜癌中突变。在这项研究中,我们揭示了SPOP通过识别MyD88中的非典型SPOP结合基序与MyD88结合并诱导其非降解性泛素化。此修饰可阻止Myddosome装配和下游NF-κB激活。SPOP在淋巴恶性肿瘤的子集中发生突变,包括弥漫性大B细胞淋巴瘤(DLBCL)。淋巴恶性肿瘤相关的SPOP突变体与MyD88的结合受损,并抑制NF-κB活化。与DLBCL相关的,结合SPOP的MyD88缺陷突变体摆脱了SPOP介导的泛素化作用,其对NF-κB活化的作用强于野生型MyD88。此外,SPOP通过抑制MyD88 /NF-κB信号传导抑制体外DLBCL细胞生长和体内肿瘤异种移植。因此,SPOP在DLBCL中起着抑癌作用。SPOP-MyD88结合界面中的突变可能会破坏SPOP-MyD88调控轴并促进DLCBL中异常的MyD88 /NF-κB活化和细胞生长。而且它们对NF-κB活化的作用要强于野生型MyD88。此外,SPOP通过抑制MyD88 /NF-κB信号传导抑制体外DLBCL细胞生长和体内肿瘤异种移植。因此,SPOP在DLBCL中起着抑癌作用。SPOP-MyD88结合界面中的突变可能会破坏SPOP-MyD88调控轴并促进DLCBL中异常的MyD88 /NF-κB活化和细胞生长。而且它们对NF-κB活化的作用要强于野生型MyD88。此外,SPOP通过抑制MyD88 /NF-κB信号传导抑制体外DLBCL细胞生长和体内肿瘤异种移植。因此,SPOP在DLBCL中起着抑癌作用。SPOP-MyD88结合界面中的突变可能会破坏SPOP-MyD88调控轴并促进DLCBL中异常的MyD88 /NF-κB活化和细胞生长。


























 京公网安备 11010802027423号
京公网安备 11010802027423号