Journal of Catalysis ( IF 7.3 ) Pub Date : 2019-11-27 , DOI: 10.1016/j.jcat.2019.11.004 Neil K. Razdan , Anurag Kumar , Brandon L. Foley , Aditya Bhan
|
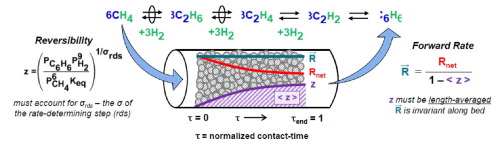
Acetylene is identified as a key intermediate in methane dehydroaromatization (DHA) reactions present in concentrations (1) Pascal. The rank of acetylene and other C2 hydrocarbon intermediates is determined by conversion-selectivity profiles collected from 0.01% to 8% methane conversion varied by extent of “non-selective” deactivation of Mo/H-ZSM-5 catalysts. Ethane is shown to be the sole primary product of methane pyrolysis and is sequentially dehydrogenated to ethylene and acetylene – which aromatizes to benzene with rates similar to direct acetylene aromatization measured in the absence of methane. The influence of CH cleavage and C
C coupling events to control the rate and reversibility of DHA is assessed by the degree of reversibility control, introduced here for the first time, and the degree of rate control. The approach to equilibrium of the methane to benzene synthesis reaction is length averaged and affinity averaged by the degree of reversibility control of each intervening elementary step to rigorously calculate forward rates of benzene synthesis by use of De Donder relations. Forward rates are found to be invariant along the catalyst bed once the DHA network reaches a pseudo-steady state and methane, ethane, and ethylene form an equilibrated pool.
中文翻译:

乙烯和乙炔对Mo / H-ZSM-5催化剂上甲烷脱氢芳构化速率和可逆性的影响
乙炔被确定为甲烷脱氢芳构化(DHA)反应的关键中间体 (1)帕斯卡。乙炔和其他C 2烃中间体的等级由转化率-选择性分布图确定,该分布图由0.01%至8%的甲烷转化率决定,甲烷转化率随Mo / H-ZSM-5催化剂的“非选择性”失活程度而变化。乙烷是甲烷热解的唯一主要产物,可依次脱氢为乙烯和乙炔,后者以类似于在不存在甲烷的情况下直接进行乙炔芳构化的速率,芳构化为苯。C H裂解和C的影响
通过首次在此介绍的可逆性控制程度和速率控制程度来评估控制DHA的速率和可逆性的C偶合事件。通过每个中间基本步骤的可逆性控制程度,对甲烷与苯合成反应达到平衡的方法进行长度平均和亲和力平均,以利用De Donder关系严格计算苯合成的正向反应速率。一旦DHA网络达到拟稳态,并且甲烷,乙烷和乙烯形成平衡池,则沿催化剂床的前进速率将保持不变。


























 京公网安备 11010802027423号
京公网安备 11010802027423号