Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Light-triggered selective ROS-dependent autophagy by bioactive nanoliposomes for efficient cancer theranostics.
Nanoscale ( IF 6.7 ) Pub Date : 2020-01-08 , DOI: 10.1039/c9nr05211a Tejaswini Appidi 1 , Deepak Bharadwaj Pemmaraju , Rafiq Ahmad Khan , Syed Baseeruddin Alvi , Rohit Srivastava , Mahadeb Pal , Nooruddin Khan , Aravind Kumar Rengan
Nanoscale ( IF 6.7 ) Pub Date : 2020-01-08 , DOI: 10.1039/c9nr05211a Tejaswini Appidi 1 , Deepak Bharadwaj Pemmaraju , Rafiq Ahmad Khan , Syed Baseeruddin Alvi , Rohit Srivastava , Mahadeb Pal , Nooruddin Khan , Aravind Kumar Rengan
Affiliation
|
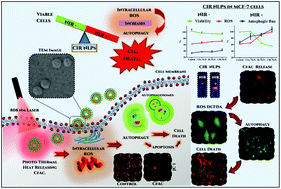
Light-responsive nanoliposomes are being reported to induce cancer cell death through heat and reactive oxygen species (ROS). Nanoliposomes (CIR NLPs) encapsulating a near-infrared (NIR) light-sensitive dye, IR780, and a bioactive chlorophyll-rich fraction of Anthocephalus cadamba (CfAc) were synthesized and characterized. These CIR NLPs, when activated by NIR light, displayed localized synergistic cancer cell death under in vitro and in vivo conditions. We demonstrated a NIR light-mediated release of CfAc in cancer cells. The bioactive CfAc was selective in causing ROS generation (leading to autophagic cell death) in cancer cells, while normal healthy cells were unaffected. An increase in the intracellular ROS leading to enhanced lipidation of microtubule-associated protein light chain 3 (LC3-II) was observed only in cancer cells, while normal cells showed no increase in either ROS or LC3-II. In vivo analysis of CIR NLPs in an orthotopic mouse model showed better anti-tumorigenic potential through a combined effect (i.e. via heat and CfAc). We reported for the first time induction of selective and localized, bioactive phyto fraction-mediated autophagic cancer cell death through an NIR light trigger. The synergistic activation of ROS-mediated autophagy by light-triggered nanoliposomes can be a useful strategy for enhancing the anticancer potential of combinational therapies.
中文翻译:

生物活性纳米脂质体对光触发的选择性ROS依赖性自噬进行有效的癌症治疗。
据报道,光响应性纳米脂质体通过热量和活性氧(ROS)诱导癌细胞死亡。合成并表征了包封近红外(NIR)感光染料IR780的纳米脂质体(CIR NLPs)和cad cadamba cadamba(CfAc)富含生物活性的叶绿素级分。这些CIR NLP在被NIR光激活时,在体外和体内条件下均表现出局部协同癌细胞死亡。我们证明了NIR光介导的癌细胞中CfAc的释放。具有生物活性的CfAc具有选择性,可在癌细胞中引起ROS生成(导致自噬细胞死亡),而正常的健康细胞不受影响。仅在癌细胞中观察到导致微管相关蛋白轻链3(LC3-II)脂质增加的细胞内ROS的增加,正常细胞的ROS或LC3-II均未增加。在原位小鼠模型中对CIR NLP的体内分析显示,通过联合作用(即通过加热和CfAc),具有更好的抗肿瘤潜力。我们首次报道了通过近红外光触发诱导选择性和局部生物活性植物级分介导的自噬癌细胞死亡。光触发的纳米脂质体对ROS介导的自噬的协同激活可能是增强联合疗法抗癌潜力的有用策略。生物活性植物分数介导的自噬癌细胞通过近红外光触发死亡。光触发的纳米脂质体对ROS介导的自噬的协同激活可能是增强联合疗法抗癌潜力的有用策略。生物活性植物分数介导的自噬癌细胞通过近红外光触发死亡。光触发的纳米脂质体对ROS介导的自噬的协同激活可能是增强联合疗法抗癌潜力的有用策略。
更新日期:2020-01-08
中文翻译:

生物活性纳米脂质体对光触发的选择性ROS依赖性自噬进行有效的癌症治疗。
据报道,光响应性纳米脂质体通过热量和活性氧(ROS)诱导癌细胞死亡。合成并表征了包封近红外(NIR)感光染料IR780的纳米脂质体(CIR NLPs)和cad cadamba cadamba(CfAc)富含生物活性的叶绿素级分。这些CIR NLP在被NIR光激活时,在体外和体内条件下均表现出局部协同癌细胞死亡。我们证明了NIR光介导的癌细胞中CfAc的释放。具有生物活性的CfAc具有选择性,可在癌细胞中引起ROS生成(导致自噬细胞死亡),而正常的健康细胞不受影响。仅在癌细胞中观察到导致微管相关蛋白轻链3(LC3-II)脂质增加的细胞内ROS的增加,正常细胞的ROS或LC3-II均未增加。在原位小鼠模型中对CIR NLP的体内分析显示,通过联合作用(即通过加热和CfAc),具有更好的抗肿瘤潜力。我们首次报道了通过近红外光触发诱导选择性和局部生物活性植物级分介导的自噬癌细胞死亡。光触发的纳米脂质体对ROS介导的自噬的协同激活可能是增强联合疗法抗癌潜力的有用策略。生物活性植物分数介导的自噬癌细胞通过近红外光触发死亡。光触发的纳米脂质体对ROS介导的自噬的协同激活可能是增强联合疗法抗癌潜力的有用策略。生物活性植物分数介导的自噬癌细胞通过近红外光触发死亡。光触发的纳米脂质体对ROS介导的自噬的协同激活可能是增强联合疗法抗癌潜力的有用策略。


























 京公网安备 11010802027423号
京公网安备 11010802027423号