当前位置:
X-MOL 学术
›
BBA Gen. Subj.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Alteration of the groove width of DNA induced by the multimodal hydrogen bonding of denaturants with DNA bases in its grooves affects their stability.
Biochimica et Biophysica Acta (BBA) - General Subjects ( IF 3 ) Pub Date : 2019-11-27 , DOI: 10.1016/j.bbagen.2019.129498 Sunipa Sarkar 1 , Prashant Chandra Singh 1
Biochimica et Biophysica Acta (BBA) - General Subjects ( IF 3 ) Pub Date : 2019-11-27 , DOI: 10.1016/j.bbagen.2019.129498 Sunipa Sarkar 1 , Prashant Chandra Singh 1
Affiliation
|
BACKGROUND
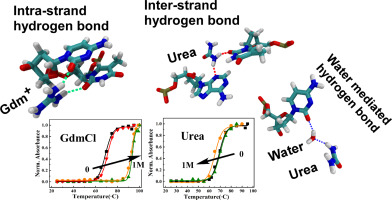
Denaturants, namely, urea and guanidinium chloride (GdmCl) affect the stability as well as structure of DNA. Critical assessment of the role of hydrogen bonding of these denaturants with the different regions of DNA is essential in terms of its stability and structural aspect. However, the understanding of the mechanistic aspects of structural change of DNA induced by the denaturants is not yet well understood.
METHODS
In this study, various spectroscopic along with molecular dynamics (MD) simulation techniques were employed to understand the role of hydrogen bonding of these denaturants with DNA bases in their stability and structural change.
RESULTS AND CONCLUSION
It has been found that both, GdmCl and urea intrude into groove region of DNA by striping surrounding water. The hydrogen bonding pattern of Gdm+ and urea with DNA bases in its groove region is multimodal and distinctly different from each other. The interaction of GdmCl with DNA is stabilized by electrostatic interaction whereas electrostatic and Lennard-Jones interactions both contribute for urea. Gdm+ forms direct hydrogen bond with the bases in the minor groove of DNA whereas direct and water assisted hydrogen bond takes place with urea. The hydrogen bond formed between Gdm+ with bases in the groove region of DNA is stronger than urea due to strong electrostatic interaction along with less self-aggregation of Gdm+ than urea. The distinct hydrogen bonding capability of Gdm+ and urea with DNA bases in its groove region affects its width differently. The interaction of Gdm+ decreases the width of the minor and major groove which probably increases the strength of hydrogen bond between the Watson-Crick base pairs of DNA leading to its stability. In contrast, the interaction of urea does not affect much to the width of the grooves except the marginal increase in the minor groove width which probably decreases the strength of hydrogen bond between Watson Crick base pairs leading to the destabilization of DNA.
GENERAL SIGNIFICANCE
Our study clearly depicts the role of hydrogen bonding between DNA bases and denaturants in their stability and structural change which can be used further for designing of the guanidinium based drug molecules.
中文翻译:

变性剂与凹槽中DNA碱基的多峰氢键引起的DNA凹槽宽度的变化会影响其稳定性。
背景技术变性剂,即尿素和氯化胍(GdmCl)影响DNA的稳定性以及结构。对于这些变性剂与DNA不同区域的氢键作用的关键评估,就其稳定性和结构方面而言至关重要。但是,对由变性剂诱导的DNA的结构变化的机理方面的理解尚不十分清楚。方法在本研究中,采用各种光谱学以及分子动力学(MD)模拟技术来了解这些变性剂与DNA碱基的氢键在其稳定性和结构变化中的作用。结果与结论已经发现,GdmCl和尿素都通过剥离周围的水而侵入DNA的凹槽区域。Gdm +和尿素在其凹槽区域具有DNA碱基的氢键模式是多峰的,并且彼此之间明显不同。GdmCl与DNA的相互作用通过静电相互作用得以稳定,而静电和Lennard-Jones相互作用均对尿素有所贡献。Gdm +与DNA小沟中的碱基形成直接氢键,而直接和水辅助的氢键与尿素发生。Gdm +与DNA凹槽区域中的碱基之间形成的氢键比尿素强,这是由于强的静电相互作用以及Gdm +的自聚集比尿素少。Gdm +和尿素在其凹槽区域具有DNA碱基的独特氢键结合能力会对其宽度产生不同的影响。Gdm +的相互作用减少了主沟和主沟的宽度,这可能会增加Watson-Crick DNA碱基对之间的氢键强度,从而导致其稳定性。相比之下,尿素的相互作用对凹槽的宽度影响不大,除了较小的凹槽宽度的边际增加,这可能会降低Watson Crick碱基对之间的氢键强度,从而导致DNA不稳定。一般意义我们的研究清楚地描述了DNA碱基和变性剂之间氢键在其稳定性和结构变化中的作用,这些作用可进一步用于设计胍基药物分子。尿素的相互作用对凹槽的宽度影响不大,除了较小的凹槽宽度的边际增加,这可能会降低Watson Crick碱基对之间的氢键强度,从而导致DNA不稳定。一般意义我们的研究清楚地描述了DNA碱基和变性剂之间氢键在其稳定性和结构变化中的作用,这些作用可进一步用于设计胍基药物分子。尿素的相互作用对凹槽的宽度影响不大,除了较小的凹槽宽度的边际增加,这可能会降低Watson Crick碱基对之间的氢键强度,从而导致DNA不稳定。一般意义我们的研究清楚地描述了DNA碱基和变性剂之间氢键在其稳定性和结构变化中的作用,这些作用可进一步用于设计胍基药物分子。
更新日期:2019-11-28
中文翻译:

变性剂与凹槽中DNA碱基的多峰氢键引起的DNA凹槽宽度的变化会影响其稳定性。
背景技术变性剂,即尿素和氯化胍(GdmCl)影响DNA的稳定性以及结构。对于这些变性剂与DNA不同区域的氢键作用的关键评估,就其稳定性和结构方面而言至关重要。但是,对由变性剂诱导的DNA的结构变化的机理方面的理解尚不十分清楚。方法在本研究中,采用各种光谱学以及分子动力学(MD)模拟技术来了解这些变性剂与DNA碱基的氢键在其稳定性和结构变化中的作用。结果与结论已经发现,GdmCl和尿素都通过剥离周围的水而侵入DNA的凹槽区域。Gdm +和尿素在其凹槽区域具有DNA碱基的氢键模式是多峰的,并且彼此之间明显不同。GdmCl与DNA的相互作用通过静电相互作用得以稳定,而静电和Lennard-Jones相互作用均对尿素有所贡献。Gdm +与DNA小沟中的碱基形成直接氢键,而直接和水辅助的氢键与尿素发生。Gdm +与DNA凹槽区域中的碱基之间形成的氢键比尿素强,这是由于强的静电相互作用以及Gdm +的自聚集比尿素少。Gdm +和尿素在其凹槽区域具有DNA碱基的独特氢键结合能力会对其宽度产生不同的影响。Gdm +的相互作用减少了主沟和主沟的宽度,这可能会增加Watson-Crick DNA碱基对之间的氢键强度,从而导致其稳定性。相比之下,尿素的相互作用对凹槽的宽度影响不大,除了较小的凹槽宽度的边际增加,这可能会降低Watson Crick碱基对之间的氢键强度,从而导致DNA不稳定。一般意义我们的研究清楚地描述了DNA碱基和变性剂之间氢键在其稳定性和结构变化中的作用,这些作用可进一步用于设计胍基药物分子。尿素的相互作用对凹槽的宽度影响不大,除了较小的凹槽宽度的边际增加,这可能会降低Watson Crick碱基对之间的氢键强度,从而导致DNA不稳定。一般意义我们的研究清楚地描述了DNA碱基和变性剂之间氢键在其稳定性和结构变化中的作用,这些作用可进一步用于设计胍基药物分子。尿素的相互作用对凹槽的宽度影响不大,除了较小的凹槽宽度的边际增加,这可能会降低Watson Crick碱基对之间的氢键强度,从而导致DNA不稳定。一般意义我们的研究清楚地描述了DNA碱基和变性剂之间氢键在其稳定性和结构变化中的作用,这些作用可进一步用于设计胍基药物分子。


























 京公网安备 11010802027423号
京公网安备 11010802027423号