当前位置:
X-MOL 学术
›
Org. Process Res. Dev.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Catalytic Intermolecular C(sp3)–H Amination with Sulfamates for the Asymmetric Synthesis of Amines
Organic Process Research & Development ( IF 3.4 ) Pub Date : 2019-11-26 , DOI: 10.1021/acs.oprd.9b00424 Ali Nasrallah 1 , Yanis Lazib 1 , Vincent Boquet 1 , Benjamin Darses 1, 2 , Philippe Dauban 1
Organic Process Research & Development ( IF 3.4 ) Pub Date : 2019-11-26 , DOI: 10.1021/acs.oprd.9b00424 Ali Nasrallah 1 , Yanis Lazib 1 , Vincent Boquet 1 , Benjamin Darses 1, 2 , Philippe Dauban 1
Affiliation
|
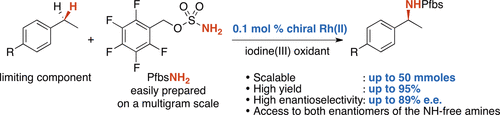
A practical catalytic asymmetric benzylic C(sp3)–H amination through the intermolecular insertion of a rhodium-bound nitrene species is reported. The reaction of various substrates (used as the limiting component) with the readily accessible sulfamate PfbsNH2 and the chiral rhodium(II) catalyst Rh2(S-tfptad)4 in the presence PhI(OPiv)2 can be performed on a multigram scale, affording the corresponding benzylic amines with high levels of efficiency and enantiocontrol. This process offers new opportunities for the asymmetric synthesis of amines.
中文翻译:

氨基磺酸酯催化分子间C(sp 3)-H胺的不对称合成
据报道,通过分子间插入铑键合的氮原子,可实现催化不对称的苄基C(sp 3)-H胺化反应。在存在PhI(OPiv)2的情况下,各种底物(用作限制组分)与易于获得的氨基磺酸盐PfbsNH 2和手性铑(II)催化剂Rh 2(S -tfptad)4的反应可以以克数进行。 ,从而提供相应的苄基胺,且具有较高的效率和对映体控制能力。该方法为胺的不对称合成提供了新的机会。
更新日期:2019-11-26
中文翻译:

氨基磺酸酯催化分子间C(sp 3)-H胺的不对称合成
据报道,通过分子间插入铑键合的氮原子,可实现催化不对称的苄基C(sp 3)-H胺化反应。在存在PhI(OPiv)2的情况下,各种底物(用作限制组分)与易于获得的氨基磺酸盐PfbsNH 2和手性铑(II)催化剂Rh 2(S -tfptad)4的反应可以以克数进行。 ,从而提供相应的苄基胺,且具有较高的效率和对映体控制能力。该方法为胺的不对称合成提供了新的机会。



























 京公网安备 11010802027423号
京公网安备 11010802027423号