Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Type I Interferon Signaling Disrupts the Hepatic Urea Cycle and Alters Systemic Metabolism to Suppress T Cell Function.
Immunity ( IF 32.4 ) Pub Date : 2019-11-26 , DOI: 10.1016/j.immuni.2019.10.014 Alexander Lercher 1 , Anannya Bhattacharya 1 , Alexandra M Popa 1 , Michael Caldera 1 , Moritz F Schlapansky 1 , Hatoon Baazim 1 , Benedikt Agerer 1 , Bettina Gürtl 1 , Lindsay Kosack 1 , Peter Májek 1 , Julia S Brunner 2 , Dijana Vitko 3 , Theresa Pinter 4 , Jakob-Wendelin Genger 1 , Anna Orlova 5 , Natalia Pikor 6 , Daniela Reil 1 , Maria Ozsvár-Kozma 7 , Ulrich Kalinke 8 , Burkhard Ludewig 6 , Richard Moriggl 9 , Keiryn L Bennett 1 , Jörg Menche 1 , Paul N Cheng 10 , Gernot Schabbauer 2 , Michael Trauner 11 , Kristaps Klavins 1 , Andreas Bergthaler 1
Immunity ( IF 32.4 ) Pub Date : 2019-11-26 , DOI: 10.1016/j.immuni.2019.10.014 Alexander Lercher 1 , Anannya Bhattacharya 1 , Alexandra M Popa 1 , Michael Caldera 1 , Moritz F Schlapansky 1 , Hatoon Baazim 1 , Benedikt Agerer 1 , Bettina Gürtl 1 , Lindsay Kosack 1 , Peter Májek 1 , Julia S Brunner 2 , Dijana Vitko 3 , Theresa Pinter 4 , Jakob-Wendelin Genger 1 , Anna Orlova 5 , Natalia Pikor 6 , Daniela Reil 1 , Maria Ozsvár-Kozma 7 , Ulrich Kalinke 8 , Burkhard Ludewig 6 , Richard Moriggl 9 , Keiryn L Bennett 1 , Jörg Menche 1 , Paul N Cheng 10 , Gernot Schabbauer 2 , Michael Trauner 11 , Kristaps Klavins 1 , Andreas Bergthaler 1
Affiliation
|
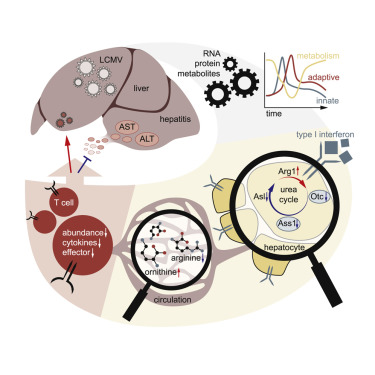
Infections induce complex host responses linked to antiviral defense, inflammation, and tissue damage and repair. We hypothesized that the liver, as a central metabolic hub, may orchestrate systemic metabolic changes during infection. We infected mice with chronic lymphocytic choriomeningitis virus (LCMV), performed RNA sequencing and proteomics of liver tissue, and integrated these data with serum metabolomics at different infection phases. Widespread reprogramming of liver metabolism occurred early after infection, correlating with type I interferon (IFN-I) responses. Viral infection induced metabolic alterations of the liver that depended on the interferon alpha/beta receptor (IFNAR1). Hepatocyte-intrinsic IFNAR1 repressed the transcription of metabolic genes, including Otc and Ass1, which encode urea cycle enzymes. This led to decreased arginine and increased ornithine concentrations in the circulation, resulting in suppressed virus-specific CD8+ T cell responses and ameliorated liver pathology. These findings establish IFN-I-induced modulation of hepatic metabolism and the urea cycle as an endogenous mechanism of immunoregulation. VIDEO ABSTRACT.
中文翻译:

I 型干扰素信号传导会扰乱肝脏尿素循环并改变全身代谢,从而抑制 T 细胞功能。
感染会诱发与抗病毒防御、炎症以及组织损伤和修复相关的复杂宿主反应。我们假设肝脏作为代谢中心,可能在感染期间协调全身代谢变化。我们用慢性淋巴细胞脉络膜脑膜炎病毒(LCMV)感染小鼠,对肝组织进行RNA测序和蛋白质组学,并将这些数据与不同感染阶段的血清代谢组学进行整合。感染后早期肝脏代谢发生广泛的重新编程,与 I 型干扰素 (IFN-I) 反应相关。病毒感染引起依赖于干扰素α/β受体(IFNAR1)的肝脏代谢改变。肝细胞固有的 IFNAR1 抑制代谢基因的转录,包括编码尿素循环酶的 Otc 和 Ass1。这导致循环中精氨酸减少和鸟氨酸浓度增加,从而抑制病毒特异性 CD8+ T 细胞反应并改善肝脏病理。这些发现确立了 IFN-I 诱导的肝代谢和尿素循环调节作为免疫调节的内源机制。视频摘要。
更新日期:2019-11-27
中文翻译:

I 型干扰素信号传导会扰乱肝脏尿素循环并改变全身代谢,从而抑制 T 细胞功能。
感染会诱发与抗病毒防御、炎症以及组织损伤和修复相关的复杂宿主反应。我们假设肝脏作为代谢中心,可能在感染期间协调全身代谢变化。我们用慢性淋巴细胞脉络膜脑膜炎病毒(LCMV)感染小鼠,对肝组织进行RNA测序和蛋白质组学,并将这些数据与不同感染阶段的血清代谢组学进行整合。感染后早期肝脏代谢发生广泛的重新编程,与 I 型干扰素 (IFN-I) 反应相关。病毒感染引起依赖于干扰素α/β受体(IFNAR1)的肝脏代谢改变。肝细胞固有的 IFNAR1 抑制代谢基因的转录,包括编码尿素循环酶的 Otc 和 Ass1。这导致循环中精氨酸减少和鸟氨酸浓度增加,从而抑制病毒特异性 CD8+ T 细胞反应并改善肝脏病理。这些发现确立了 IFN-I 诱导的肝代谢和尿素循环调节作为免疫调节的内源机制。视频摘要。


























 京公网安备 11010802027423号
京公网安备 11010802027423号