当前位置:
X-MOL 学术
›
Catal. Sci. Technol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Insight into the reaction mechanism and chemoselectivity in the cycloaddition of ynamides and isoxazoles with H2O
Catalysis Science & Technology ( IF 5 ) Pub Date : 2019-11-27 , DOI: 10.1039/c9cy01964b Lin Zhou 1, 2, 3, 4, 5 , Li Yang 1, 2, 3, 4, 5 , Songshan Dai 1, 2, 3, 4, 5 , Yuanyuan Gao 1, 2, 3, 4, 5 , Ran Fang 1, 2, 3, 4, 5 , Alexander M. Kirillov 6, 7, 8, 9, 10 , Lizi Yang 1, 2, 3, 4, 5
Catalysis Science & Technology ( IF 5 ) Pub Date : 2019-11-27 , DOI: 10.1039/c9cy01964b Lin Zhou 1, 2, 3, 4, 5 , Li Yang 1, 2, 3, 4, 5 , Songshan Dai 1, 2, 3, 4, 5 , Yuanyuan Gao 1, 2, 3, 4, 5 , Ran Fang 1, 2, 3, 4, 5 , Alexander M. Kirillov 6, 7, 8, 9, 10 , Lizi Yang 1, 2, 3, 4, 5
Affiliation
|
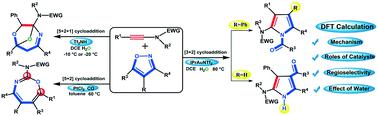
The mechanism and chemoselectivity in the cycloaddition of ynamides and isoxazoles have been explored by the density functional theory (DFT) in model systems composed of a Brønsted acid (HNTf2), gold(I) [IPrAuNTf2] or platinum(II) (PtCl2/CO) catalyst, either with or without the presence of H2O. The DFT calculations reveal that all these catalysts entail similar nucleophilic attack of isoxazole on the catalyst-ligated ynamide forming a vinyl intermediate, which can isomerize to an α-imino intermediate upon cleavage of the isoxazole N–O bond. The completely distinct reaction pathways are observed after the formation of the α-imino intermediate. For the Brønsted acid catalyst, [5 + 2 + 1] cycloaddition with H2O is the favorable way to generate O-bridged tetrahydro-1,4-oxazepines. If the Brønsted acid is replaced by a gold(I) catalyst, a [3 + 2] cycloaddition product is produced, either in the absence or in the presence of H2O. Regarding the Pt(II) catalyst, 1,3-oxazepines are formed through [5 + 2] annulation. Furthermore, the [5 + 2] annulation product in this Pt(II)-catalyzed system can also be predicted upon addition of H2O. The unique properties of the three selected catalysts were explored in detail through distortion/interaction analysis. The obtained theoretical data account for an observed disparate product formation when using three catalytic systems and provide a theoretical foundation to choose the optimal catalyst for the title reaction. These results can be of particular significance for synthetic chemists toward the design of catalytic systems and cycloaddition transformations involving ynamides, isoxazoles and related derivatives.
中文翻译:

深入了解酰胺与异恶唑与H2O环加成的反应机理和化学选择性
通过密度泛函理论(DFT)在由Brønsted酸(HNTf 2),金(I)[IPrAuNTf 2 ]或铂(II)(PtCl 2 / CO)催化剂,无论是否存在H 2O. DFT计算表明,所有这些催化剂都需要异恶唑对与催化剂连接的乙酰胺进行类似的亲核攻击,形成乙烯基中间体,该异氰酸酯在异恶唑N–O键断裂时可以异构化为α-亚氨基中间体。在形成α-亚氨基中间体之后,观察到完全不同的反应途径。对于布朗斯台德酸催化剂,用H 2 O进行[5 + 2 +1]环加成反应是生成O桥联的四氢-1,4-氧杂氮杂烷的有利方法。如果布朗斯台德酸是由金(代替我)催化剂,[3 + 2]环加成产物产生时,无论是在不存在或存在H的存在2 O.关于铂(II)催化剂,通过[5 + 2]环化反应形成1,3-氧杂氮平。此外,还可以通过添加H 2 O预测此Pt(II)催化体系中的[5 + 2]环化产物。通过变形/相互作用分析详细研究了三种所选催化剂的独特性质。当使用三个催化体系时,获得的理论数据说明了观察到的完全不同的产物形成,并为选择最佳催化剂进行标题反应提供了理论基础。这些结果对于合成化学家对涉及酰胺,异恶唑和相关衍生物的催化体系设计和环加成转化特别重要。
更新日期:2019-11-27
中文翻译:

深入了解酰胺与异恶唑与H2O环加成的反应机理和化学选择性
通过密度泛函理论(DFT)在由Brønsted酸(HNTf 2),金(I)[IPrAuNTf 2 ]或铂(II)(PtCl 2 / CO)催化剂,无论是否存在H 2O. DFT计算表明,所有这些催化剂都需要异恶唑对与催化剂连接的乙酰胺进行类似的亲核攻击,形成乙烯基中间体,该异氰酸酯在异恶唑N–O键断裂时可以异构化为α-亚氨基中间体。在形成α-亚氨基中间体之后,观察到完全不同的反应途径。对于布朗斯台德酸催化剂,用H 2 O进行[5 + 2 +1]环加成反应是生成O桥联的四氢-1,4-氧杂氮杂烷的有利方法。如果布朗斯台德酸是由金(代替我)催化剂,[3 + 2]环加成产物产生时,无论是在不存在或存在H的存在2 O.关于铂(II)催化剂,通过[5 + 2]环化反应形成1,3-氧杂氮平。此外,还可以通过添加H 2 O预测此Pt(II)催化体系中的[5 + 2]环化产物。通过变形/相互作用分析详细研究了三种所选催化剂的独特性质。当使用三个催化体系时,获得的理论数据说明了观察到的完全不同的产物形成,并为选择最佳催化剂进行标题反应提供了理论基础。这些结果对于合成化学家对涉及酰胺,异恶唑和相关衍生物的催化体系设计和环加成转化特别重要。



























 京公网安备 11010802027423号
京公网安备 11010802027423号