Mucosal Immunology ( IF 8 ) Pub Date : 2019-11-26 , DOI: 10.1038/s41385-019-0233-6 Sharmin Begum 1 , France Moreau 1 , Aralia Leon Coria 1 , Kris Chadee 1
|
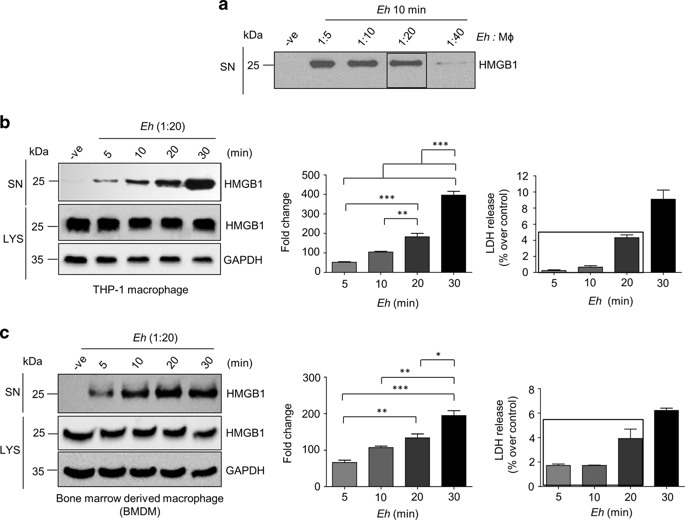
Even though Entamoeba histolytica (Eh)-induced host pro-inflammatory responses play a critical role in disease, we know very little about the host factors that regulate this response. Direct contact between host cell and Eh signify the highest level of danger, and to eliminate this threat, the host immune system elicits an augmented immune response. To understand the mechanisms of this response, we investigated the induction and release of the endogenous alarmin molecule high-mobility group box 1 (HMGB1) that act as a pro-inflammatory cytokine and chemoattractant during Eh infection. Eh in contact with macrophage induced a dose- and time-dependent secretion of HMGB1 in the absence of cell death. Secretion of HMGB1 was facilitated by Eh surface Gal-lectin-activated phosphoinositide 3-kinase and nuclear factor-κB signaling and up-regulation of histone acetyltransferase activity to trigger acetylated HMGB1 translocation from the nucleus. Unlike lipopolysaccharide, Eh-induced HMGB1 release was independent of caspase-1-mediated inflammasome and gasdermin D pores. In vivo, Eh inoculation in specific pathogen-free but not germ-free mice was associated with high levels of pro-inflammatory cytokines such as tumor necrosis factor-α, interleukin-1β, and keratinocyte-derived chemokine, which was suppressed with HMGB1 neutralization. This study reveals that Eh-induced active secretion of the HMGB1 plays a key role in shaping the pro-inflammatory landscape critical in innate host defense against amebiasis.
中文翻译:

溶组织内阿米巴刺激来自巨噬细胞的警报分子 HMGB1 以增强先天宿主防御
尽管溶组织内阿米巴( Eh ) 诱导的宿主促炎症反应在疾病中起着关键作用,但我们对调节这种反应的宿主因素知之甚少。宿主细胞和Eh之间的直接接触意味着最高级别的危险,为了消除这种威胁,宿主免疫系统会引发增强的免疫反应。为了解这种反应的机制,我们研究了内源性警报分子高迁移率族盒 1 (HMGB1) 的诱导和释放,该分子在Eh感染期间充当促炎细胞因子和趋化剂。嗯在没有细胞死亡的情况下,与巨噬细胞接触会诱导 HMGB1 的剂量和时间依赖性分泌。Eh表面半乳糖凝集素激活的磷酸肌醇 3-激酶和核因子-κB 信号以及组蛋白乙酰转移酶活性的上调促进了 HMGB1 的分泌,以触发乙酰化 HMGB1 从细胞核易位。与脂多糖不同,Eh诱导的 HMGB1 释放与 caspase-1 介导的炎性体和 gasdermin D 孔无关。在体内,呃在无特定病原体但非无菌小鼠中接种与高水平的促炎细胞因子相关,例如肿瘤坏死因子-α、白介素-1β 和角质形成细胞衍生的趋化因子,这些因子被 HMGB1 中和抑制。这项研究表明,Eh诱导的 HMGB1 主动分泌在形成促炎景观方面起着关键作用,而促炎景观对于先天宿主防御阿米巴病至关重要。



























 京公网安备 11010802027423号
京公网安备 11010802027423号