当前位置:
X-MOL 学术
›
ChemMedChem
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Identification of Phenylphthalazinones as a New Class of Leishmania infantum Inhibitors.
ChemMedChem ( IF 3.4 ) Pub Date : 2019-11-22 , DOI: 10.1002/cmdc.201900538 Maarten Sijm 1 , Erik de Heuvel 1 , An Matheeussen 2 , Guy Caljon 2 , Louis Maes 2 , Geert-Jan Sterk 1 , Iwan J P de Esch 1 , Rob Leurs 1
ChemMedChem ( IF 3.4 ) Pub Date : 2019-11-22 , DOI: 10.1002/cmdc.201900538 Maarten Sijm 1 , Erik de Heuvel 1 , An Matheeussen 2 , Guy Caljon 2 , Louis Maes 2 , Geert-Jan Sterk 1 , Iwan J P de Esch 1 , Rob Leurs 1
Affiliation
|
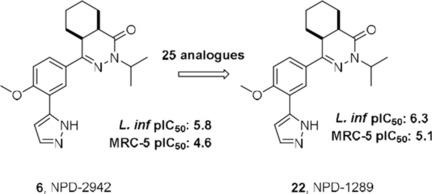
Leishmaniasis is a neglected parasitic disease caused by over 20 different Leishmania species. Current treatments often rely on harsh regimes of pentavalent antimonials such as sodium stibogluconate, while more recent drugs suffer other shortcomings such as low stability and rapid emergence of treatment failure, amongst others. Furthermore, the effectiveness of drugs varies depending on the infecting Leishmania species, thus there is an urgent need for new and effective anti-leishmanial drugs. Screening of an in-house compound library identified the hexahydrophthalazinone NPD-2942 as a low micromolar hit with a pIC50 of 5.8 against L. infantum and a pIC50 of 4.6 for cytotoxicity against human MRC-5 fibroblasts. To derive structure-activity relationships, we modified the cyclohexyl ring of the hexahydrophthalazinone scaffold and 1,2,3-triazoles were attempted as replacement for the pyrazole ring, amongst others. Ultimately, the 2,3-pyrazole-substituted hexahydrophthalazinone NPD-1289 was identified as the most potent analogue in this series with a pIC50 of 6.3, although some cytotoxicity toward MRC-5 cells (pIC50 =5.1) was recorded as well. Replacement of the unsubstituted 2,3-pyrazole with 1,2,3-triazoles led to compounds with lower anti-leishmanial activity. The current scaffold is a valuable new starting point for optimization toward novel anti-leishmanial drugs.
中文翻译:

苯邻二氮杂壬酮作为一种新的婴儿利什曼原虫抑制剂的鉴定。
利什曼病是由20多种不同的利什曼原虫物种引起的被忽视的寄生虫病。当前的治疗通常依赖于五价锑的苛刻方案,例如司他葡糖酸钠,而最近的药物还具有其他缺点,例如稳定性低和治疗失败迅速出现。此外,药物的有效性根据感染的利什曼原虫种类而变化,因此迫切需要新的有效的抗利什曼病药物。内部化合物库的筛选确定了六氢酞嗪酮NPD-2942是低微摩尔分子,对婴儿乳杆菌的pIC50为5.8,对人MRC-5成纤维细胞的细胞毒性的pIC50为4.6。为了得出构效关系,我们修饰了六氢酞嗪酮支架的环己基环和1,2,尝试使用3-三唑代替吡唑环。最终,尽管也记录了对MRC-5细胞的某些细胞毒性(pIC50 = 5.1),但2,3-吡唑取代的六氢酞嗪酮NPD-1289被确定为该系列中最有效的类似物,pIC50为6.3。用1,2,3-三唑取代未取代的2,3-吡唑会导致化合物的抗leishmanial活性较低。当前的支架是优化新型抗利什曼病毒药物的有价值的新起点。用1,2,3-三唑取代未取代的2,3-吡唑会导致化合物的抗leishmanial活性较低。当前的支架是优化新型抗利什曼病毒药物的有价值的新起点。用1,2,3-三唑取代未取代的2,3-吡唑会导致化合物的抗leishmanial活性较低。当前的支架是优化新型抗利什曼病毒药物的有价值的新起点。
更新日期:2019-12-09
中文翻译:

苯邻二氮杂壬酮作为一种新的婴儿利什曼原虫抑制剂的鉴定。
利什曼病是由20多种不同的利什曼原虫物种引起的被忽视的寄生虫病。当前的治疗通常依赖于五价锑的苛刻方案,例如司他葡糖酸钠,而最近的药物还具有其他缺点,例如稳定性低和治疗失败迅速出现。此外,药物的有效性根据感染的利什曼原虫种类而变化,因此迫切需要新的有效的抗利什曼病药物。内部化合物库的筛选确定了六氢酞嗪酮NPD-2942是低微摩尔分子,对婴儿乳杆菌的pIC50为5.8,对人MRC-5成纤维细胞的细胞毒性的pIC50为4.6。为了得出构效关系,我们修饰了六氢酞嗪酮支架的环己基环和1,2,尝试使用3-三唑代替吡唑环。最终,尽管也记录了对MRC-5细胞的某些细胞毒性(pIC50 = 5.1),但2,3-吡唑取代的六氢酞嗪酮NPD-1289被确定为该系列中最有效的类似物,pIC50为6.3。用1,2,3-三唑取代未取代的2,3-吡唑会导致化合物的抗leishmanial活性较低。当前的支架是优化新型抗利什曼病毒药物的有价值的新起点。用1,2,3-三唑取代未取代的2,3-吡唑会导致化合物的抗leishmanial活性较低。当前的支架是优化新型抗利什曼病毒药物的有价值的新起点。用1,2,3-三唑取代未取代的2,3-吡唑会导致化合物的抗leishmanial活性较低。当前的支架是优化新型抗利什曼病毒药物的有价值的新起点。


























 京公网安备 11010802027423号
京公网安备 11010802027423号