当前位置:
X-MOL 学术
›
ACS Chem. Biol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Synthetic Antibody Binding to a Preorganized RNA Domain of Hepatitis C Virus Internal Ribosome Entry Site Inhibits Translation.
ACS Chemical Biology ( IF 4 ) Pub Date : 2019-12-10 , DOI: 10.1021/acschembio.9b00785 Deepak Koirala 1 , Anna Lewicka 1 , Yelena Koldobskaya 1 , Hao Huang 1 , Joseph A Piccirilli 1, 2
ACS Chemical Biology ( IF 4 ) Pub Date : 2019-12-10 , DOI: 10.1021/acschembio.9b00785 Deepak Koirala 1 , Anna Lewicka 1 , Yelena Koldobskaya 1 , Hao Huang 1 , Joseph A Piccirilli 1, 2
Affiliation
|
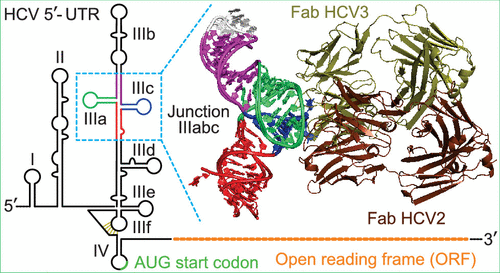
Structured RNA elements within the internal ribosome entry site (IRES) of hepatitis C virus (HCV) genome hijack host cell machinery for translation initiation through a cap-independent mechanism. Here, using a phage display selection, we obtained two antibody fragments (Fabs), HCV2 and HCV3, against HCV IRES that bind the RNA with dissociation constants of 32 ± 7 nM and 37 ± 8 nM respectively, specifically recognizing the so-called junction IIIabc (JIIIabc). We used these Fabs as crystallization chaperones and determined the high-resolution crystal structures of JIIIabc-HCV2 and -HCV3 complexes at 1.81 Å and 2.75 Å resolution respectively, revealing an antiparallel four-way junction with the IIIa and IIIc subdomains brought together through tertiary interactions. The RNA conformation observed in the structures supports the structural model for this region derived from cryo-EM data for the HCV IRES-40S ribosome complex, suggesting that the tertiary fold of the RNA preorganizes the domain for interactions with the 40S ribosome. Strikingly, both Fabs and the ribosomal protein eS27 not only interact with a common subset of nucleotides within the JIIIabc but also use physiochemically similar sets of protein residues to do so, suggesting that the RNA surface is well-suited for interactions with proteins, perhaps analogous to the "hot spot" concept elaborated for protein-protein interactions. Using a rabbit reticulocyte lysate-based translation assay with a bicistronic reporter construct, we further demonstrated that Fabs HCV2 and HCV3 specifically inhibit the HCV IRES-directed translation, implicating disruption of the JIIIabc-ribosome interaction as a potential therapeutic strategy against HCV.
中文翻译:

合成抗体与丙型肝炎病毒内部核糖体进入位点的预组织RNA域的结合会抑制翻译。
丙型肝炎病毒(HCV)基因组劫持宿主细胞机械内部核糖体进入位点(IRES)中的结构化RNA元件可通过不依赖帽的机制进行翻译起始。在这里,通过噬菌体展示选择,我们获得了针对HCV IRES的两个抗体片段(Fabs)HCV2和HCV3,它们分别以32±7 nM和37±8 nM的解离常数结合RNA,专门识别所谓的连接IIIabc(JIIIabc)。我们将这些Fabs用作结晶分子伴侣,并分别以1.81Å和2.75Å分辨率确定了JIIIabc-HCV2和-HCV3复合物的高分辨率晶体结构,揭示了通过三级相互作用将IIIa和IIIc子域反平行的四向连接。在结构中观察到的RNA构象支持了该区域的结构模型,该结构模型是从HCV IRES-40S核糖体复合物的冷冻EM数据得出的,表明RNA的三级折叠为与40S核糖体相互作用的结构域进行了预组织。令人惊讶的是,Fabs和核糖体蛋白eS27不仅与JIIIabc中的一个共同的核苷酸子集相互作用,而且还使用理化上相似的蛋白质残基集进行相互作用,这表明RNA表面非常适合与蛋白质相互作用,也许是相似的阐述了蛋白质间相互作用的“热点”概念。使用带有双顺反子报告基因构建体的兔网织红细胞裂解物为基础的翻译测定,我们进一步证明Fabs HCV2和HCV3特异性抑制HCV IRES定向的翻译,
更新日期:2019-12-11
中文翻译:

合成抗体与丙型肝炎病毒内部核糖体进入位点的预组织RNA域的结合会抑制翻译。
丙型肝炎病毒(HCV)基因组劫持宿主细胞机械内部核糖体进入位点(IRES)中的结构化RNA元件可通过不依赖帽的机制进行翻译起始。在这里,通过噬菌体展示选择,我们获得了针对HCV IRES的两个抗体片段(Fabs)HCV2和HCV3,它们分别以32±7 nM和37±8 nM的解离常数结合RNA,专门识别所谓的连接IIIabc(JIIIabc)。我们将这些Fabs用作结晶分子伴侣,并分别以1.81Å和2.75Å分辨率确定了JIIIabc-HCV2和-HCV3复合物的高分辨率晶体结构,揭示了通过三级相互作用将IIIa和IIIc子域反平行的四向连接。在结构中观察到的RNA构象支持了该区域的结构模型,该结构模型是从HCV IRES-40S核糖体复合物的冷冻EM数据得出的,表明RNA的三级折叠为与40S核糖体相互作用的结构域进行了预组织。令人惊讶的是,Fabs和核糖体蛋白eS27不仅与JIIIabc中的一个共同的核苷酸子集相互作用,而且还使用理化上相似的蛋白质残基集进行相互作用,这表明RNA表面非常适合与蛋白质相互作用,也许是相似的阐述了蛋白质间相互作用的“热点”概念。使用带有双顺反子报告基因构建体的兔网织红细胞裂解物为基础的翻译测定,我们进一步证明Fabs HCV2和HCV3特异性抑制HCV IRES定向的翻译,



























 京公网安备 11010802027423号
京公网安备 11010802027423号