当前位置:
X-MOL 学术
›
Lancet Oncol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Atezolizumab for children and young adults with previously treated solid tumours, non-Hodgkin lymphoma, and Hodgkin lymphoma (iMATRIX): a multicentre phase 1-2 study.
The Lancet Oncology ( IF 51.1 ) Pub Date : 2019-11-25 , DOI: 10.1016/s1470-2045(19)30693-x Birgit Geoerger 1 , C Michel Zwaan 2 , Lynley V Marshall 3 , Jean Michon 4 , Franck Bourdeaut 4 , Michela Casanova 5 , Nadège Corradini 6 , Gianluca Rossato 7 , Mufiza Farid-Kapadia 8 , Colby S Shemesh 9 , Katherine E Hutchinson 9 , Francis Donaldson 10 , Minlei Liao 9 , Hubert Caron 7 , Tanya Trippett 11
The Lancet Oncology ( IF 51.1 ) Pub Date : 2019-11-25 , DOI: 10.1016/s1470-2045(19)30693-x Birgit Geoerger 1 , C Michel Zwaan 2 , Lynley V Marshall 3 , Jean Michon 4 , Franck Bourdeaut 4 , Michela Casanova 5 , Nadège Corradini 6 , Gianluca Rossato 7 , Mufiza Farid-Kapadia 8 , Colby S Shemesh 9 , Katherine E Hutchinson 9 , Francis Donaldson 10 , Minlei Liao 9 , Hubert Caron 7 , Tanya Trippett 11
Affiliation
|
BACKGROUND
Atezolizumab is an inhibitor of PD-L1, which can lead to enhanced anticancer T-cell activity. We aimed to evaluate the safety, pharmacokinetics, and activity of atezolizumab in children and young adults with refractory or relapsed solid tumours, with known or expected PD-L1 expression.
METHODS
iMATRIX was a multicentre, open-label, phase 1-2 trial of patients (aged <30 years) with solid tumours or lymphomas recruited from 28 hospitals in ten countries (USA, France, Italy, UK, Spain, the Netherlands, Denmark, Israel, Switzerland, and Germany). Eligible patients younger than 18 years received 15 mg/kg atezolizumab (maximum 1200 mg); patients aged 18-29 years received the adult dose (1200 mg) until disease progression or loss of clinical benefit. Co-primary endpoints were safety (assessed by incidence of adverse events) and pharmacokinetics (assessed by serum atezolizumab concentrations). Secondary endpoints included the proportion of patients achieving an objective response. This trial is registered with ClinicalTrials.gov, number NCT02541604.
FINDINGS
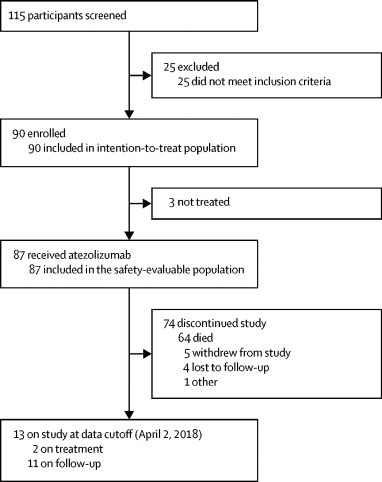
Between Nov 5, 2015, and April 2, 2018, we screened 115 patients, 25 of whom did not meet the inclusion criteria. 90 patients, with a median age of 14 years (IQR 10-17), were enrolled. At the data cutoff (April 2, 2018), two patients remained on study treatment. 87 (97%) of 90 patients received at least one dose of atezolizumab at 15 mg/kg or 1200 mg and were evaluable for safety. Three patients were not treated owing to either poor clinical condition or withdrawal of consent. In the safety-evaluable population (n=87), the most common adverse events were pyrexia (36 [41%] patients) and fatigue (31 [36%]). The most common grade 3-4 adverse event was anaemia (19 [22%] patients). The most commonly reported serious adverse events were in the categories of infections and infestations; pyrexia was the only serious adverse event reported in more than two patients. 57 (66%) patients had at least one treatment-related adverse event (grade 1-4); fatigue was the most common treatment-related adverse event (17 patients [20%]). There were no fatal adverse events. Mean serum concentrations of atezolizumab were overlapping and comparable between children receiving 15 mg/kg and young adults receiving 1200 mg of atezolizumab every 3 weeks. Serum concentrations of atezolizumab were above the target exposure level in all patients. At 6 months, four patients (5%) achieved an objective response (all partial responses).
INTERPRETATION
Although response to atezolizumab was restricted, atezolizumab was well tolerated with generally comparable exposure across populations. Our findings might help to define future development strategies for immune checkpoint inhibitors either by focusing research to specific disease subpopulations that exhibit greater benefit from immune checkpoint inhibitors, or by providing the means to identify therapeutic combination partners that augment T-cell infiltration and proliferation in so-called immune cold tumour microenvironments.
FUNDING
F Hoffmann-La Roche.
中文翻译:

Atezolizumab适用于先前治疗过的实体瘤,非霍奇金淋巴瘤和霍奇金淋巴瘤(iMATRIX)的儿童和年轻人:一项多中心1-2期研究。
背景技术Atezolizumab是PD-L1的抑制剂,可导致增强的抗癌T细胞活性。我们的目的是评估已知或预期PD-L1表达的难治性或复发性实体瘤儿童和青年成人中atezolizumab的安全性,药代动力学和活性。方法iMATRIX是一项多中心,开放性,1-2期临床试验,对象是从十个国家(美国,法国,意大利,英国,西班牙,荷兰,荷兰, ,以色列,瑞士和德国)。年龄小于18岁的合格患者接受了15 mg / kg阿妥珠单抗(最大1200 mg); 18-29岁的患者接受成人剂量(1200毫克)直至疾病进展或失去临床益处。共同主要终点是安全性(通过不良事件的发生率评估)和药代动力学(通过血清阿妥珠单抗浓度评估)。次要终点包括达到客观反应的患者比例。该试验已在ClinicalTrials.gov上注册,编号为NCT02541604。结果在2015年11月5日至2018年4月2日之间,我们筛选了115位患者,其中25位不符合纳入标准。纳入90位患者,中位年龄为14岁(IQR 10-17)。在数据截止日(2018年4月2日),两名患者仍接受研究治疗。90名患者中有87名(97%)接受了至少一种剂量的15 mg / kg或1200 mg的atezolizumab,并且可以评估其安全性。三名患者由于临床状况不佳或撤回同意书而未得到治疗。在可安全评估的人群中(n = 87),最常见的不良事件是发热(36 [41%]患者)和疲劳(31 [36%])。最常见的3-4级不良事件是贫血(19 [22%]位患者)。最常见的严重不良事件是感染和感染。发热是超过两名患者中报告的唯一严重不良事件。57名(66%)患者患有至少一种与治疗有关的不良事件(1-4级);疲劳是最常见的治疗相关不良事件(17例[20%])。没有致命的不良事件。每3周接受15 mg / kg的儿童和接受1200 mg atezolizumab的年轻人之间,atezolizumab的平均血清浓度重叠且相当。在所有患者中,atezolizumab的血清浓度均高于目标暴露水平。在六个月后 4名患者(5%)达到了客观缓解(所有部分缓解)。解释尽管对atezolizumab的反应受到限制,但是atezolizumab的耐受性良好,总体人群中的暴露水平相当。我们的发现可能通过将研究重点放在表现出免疫检查点抑制剂更大益处的特定疾病亚群上,或通过提供手段来确定可增强T细胞浸润和增殖的治疗性组合伴侣的方式,来帮助确定免疫检查点抑制剂的未来发展策略。所谓的免疫性冷肿瘤微环境。资助F霍夫曼-拉罗什。我们的发现可能通过将研究重点放在表现出免疫检查点抑制剂更大益处的特定疾病亚群上,或通过提供手段来确定可增强T细胞浸润和增殖的治疗性组合伴侣的方式,来帮助确定免疫检查点抑制剂的未来发展策略。所谓的免疫性冷肿瘤微环境。资助霍夫曼-拉罗什(F Hoffmann-La Roche)。我们的发现可能通过将研究重点放在表现出免疫检查点抑制剂更大益处的特定疾病亚群上,或通过提供手段来确定可增强T细胞浸润和增殖的治疗性组合伴侣的方式,来帮助确定免疫检查点抑制剂的未来发展策略。所谓的免疫性冷肿瘤微环境。资助霍夫曼-拉罗什(F Hoffmann-La Roche)。
更新日期:2020-01-04
中文翻译:

Atezolizumab适用于先前治疗过的实体瘤,非霍奇金淋巴瘤和霍奇金淋巴瘤(iMATRIX)的儿童和年轻人:一项多中心1-2期研究。
背景技术Atezolizumab是PD-L1的抑制剂,可导致增强的抗癌T细胞活性。我们的目的是评估已知或预期PD-L1表达的难治性或复发性实体瘤儿童和青年成人中atezolizumab的安全性,药代动力学和活性。方法iMATRIX是一项多中心,开放性,1-2期临床试验,对象是从十个国家(美国,法国,意大利,英国,西班牙,荷兰,荷兰, ,以色列,瑞士和德国)。年龄小于18岁的合格患者接受了15 mg / kg阿妥珠单抗(最大1200 mg); 18-29岁的患者接受成人剂量(1200毫克)直至疾病进展或失去临床益处。共同主要终点是安全性(通过不良事件的发生率评估)和药代动力学(通过血清阿妥珠单抗浓度评估)。次要终点包括达到客观反应的患者比例。该试验已在ClinicalTrials.gov上注册,编号为NCT02541604。结果在2015年11月5日至2018年4月2日之间,我们筛选了115位患者,其中25位不符合纳入标准。纳入90位患者,中位年龄为14岁(IQR 10-17)。在数据截止日(2018年4月2日),两名患者仍接受研究治疗。90名患者中有87名(97%)接受了至少一种剂量的15 mg / kg或1200 mg的atezolizumab,并且可以评估其安全性。三名患者由于临床状况不佳或撤回同意书而未得到治疗。在可安全评估的人群中(n = 87),最常见的不良事件是发热(36 [41%]患者)和疲劳(31 [36%])。最常见的3-4级不良事件是贫血(19 [22%]位患者)。最常见的严重不良事件是感染和感染。发热是超过两名患者中报告的唯一严重不良事件。57名(66%)患者患有至少一种与治疗有关的不良事件(1-4级);疲劳是最常见的治疗相关不良事件(17例[20%])。没有致命的不良事件。每3周接受15 mg / kg的儿童和接受1200 mg atezolizumab的年轻人之间,atezolizumab的平均血清浓度重叠且相当。在所有患者中,atezolizumab的血清浓度均高于目标暴露水平。在六个月后 4名患者(5%)达到了客观缓解(所有部分缓解)。解释尽管对atezolizumab的反应受到限制,但是atezolizumab的耐受性良好,总体人群中的暴露水平相当。我们的发现可能通过将研究重点放在表现出免疫检查点抑制剂更大益处的特定疾病亚群上,或通过提供手段来确定可增强T细胞浸润和增殖的治疗性组合伴侣的方式,来帮助确定免疫检查点抑制剂的未来发展策略。所谓的免疫性冷肿瘤微环境。资助F霍夫曼-拉罗什。我们的发现可能通过将研究重点放在表现出免疫检查点抑制剂更大益处的特定疾病亚群上,或通过提供手段来确定可增强T细胞浸润和增殖的治疗性组合伴侣的方式,来帮助确定免疫检查点抑制剂的未来发展策略。所谓的免疫性冷肿瘤微环境。资助霍夫曼-拉罗什(F Hoffmann-La Roche)。我们的发现可能通过将研究重点放在表现出免疫检查点抑制剂更大益处的特定疾病亚群上,或通过提供手段来确定可增强T细胞浸润和增殖的治疗性组合伴侣的方式,来帮助确定免疫检查点抑制剂的未来发展策略。所谓的免疫性冷肿瘤微环境。资助霍夫曼-拉罗什(F Hoffmann-La Roche)。


























 京公网安备 11010802027423号
京公网安备 11010802027423号