Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Noncompletion and nonpublication of trials studying rare diseases: A cross-sectional analysis.
PLOS Medicine ( IF 15.8 ) Pub Date : 2019-11-21 , DOI: 10.1371/journal.pmed.1002966 Chris A Rees 1, 2 , Natalie Pica 3 , Michael C Monuteaux 1, 2 , Florence T Bourgeois 1, 2, 4
PLOS Medicine ( IF 15.8 ) Pub Date : 2019-11-21 , DOI: 10.1371/journal.pmed.1002966 Chris A Rees 1, 2 , Natalie Pica 3 , Michael C Monuteaux 1, 2 , Florence T Bourgeois 1, 2, 4
Affiliation
|
BACKGROUND
Rare diseases affect as many as 60 million people in the United States and Europe. However, most rare diseases lack effective therapies and are in critical need of clinical research. Our objective was to determine the frequency of noncompletion and nonpublication of trials studying rare diseases.
METHODS AND FINDINGS
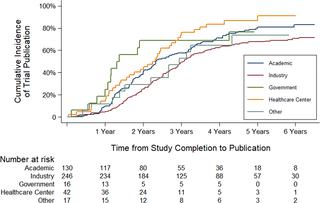
We conducted a cross-sectional analysis of randomized clinical trials studying rare diseases as defined by the Genetic and Rare Disease Information Center database that were registered in ClinicalTrials.gov between January 1, 2010, and December 31, 2012, and completed or discontinued by December 31, 2014. Our main outcome measures were the frequency of trial noncompletion and, among completed studies, frequency of trial nonpublication at 2 and 4 years following trial completion. Reasons for discontinuation were extracted from the registry, and trial sponsors were contacted for additional information, as needed. Two independent investigators performed publication searches for each trial in PubMed, EMBASE, and GoogleScholar, allowing for a minimum of 45 months between trial completion and publication. When a publication could not be identified, trial sponsors were contacted to confirm publication status. The impact of funding source on trial noncompletion was assessed with multivariable logistic regression, and the effect on time to publication was examined with Cox proportional hazards regression. Control variables included intervention type, trial phase, masking, enrollment, and study population. We analyzed 659 rare disease trials accounting for 70,305 enrolled patients. Industry was the primary funder for 327 trials (49.6%) and academic institutions for 184 trials (27.9%). There were 79 trials (12.0%) focused on pediatric populations. A total of 199 trials (30.2%) were discontinued. Lack of patient accrual (n = 64, 32.1%) and informative termination (n = 41, 20.6%) were the most common reasons for trial noncompletion. Among completed trials, 306 (66.5%) remained unpublished at 2 years and 142 (31.5%) at 4 years. In multivariable analyses, industry-funded trials were less likely to be discontinued than trials funded by healthcare centers (odds ratio [OR] 2.42; 95% confidence interval [CI] 1.34-4.39, P = 0.003). We found no significant association between funding source and time to publication. A total of 18,148 patients were enrolled in trials that were discontinued or unpublished 4 years after completion. A potential limitation of our study is that certain interventional trials for rare diseases may not have been registered in ClinicalTrials.gov, in particular Phase 0 and Phase I trials, which are not required to be registered.
CONCLUSIONS
In this study, over half of clinical trials initiated for rare diseases were either discontinued or not published 4 years after completion, resulting in large numbers of patients with rare diseases exposed to interventions that did not lead to informative findings. Concerted efforts are needed to ensure that participation of patients in rare disease trials advances scientific knowledge and treatments for rare diseases.
中文翻译:

研究罕见疾病的试验的非完成和非公开:横断面分析。
背景技术在美国和欧洲,罕见病影响多达6000万人。但是,大多数罕见疾病缺乏有效的治疗方法,因此急需临床研究。我们的目标是确定研究罕见疾病的未完成和未发表的频率。方法和结果我们对随机临床试验进行了横断面分析,该试验研究了由遗传与稀有疾病信息中心数据库定义的稀有疾病,该数据库已于2010年1月1日至2012年12月31日在ClinicalTrials.gov中注册,并已完成或在2014年12月31日停止提供服务。我们的主要结局指标是试验未完成的频率,以及在完成的研究中,试验完成后2年和4年的试验未公开的频率。从注册中心中提取了中止的原因,并根据需要与试验申办者联系以获取更多信息。两名独立研究人员针对PubMed,EMBASE和GoogleScholar中的每个试验进行了出版物检索,从试验完成到发表之间至少需要45个月的时间。当无法确定出版物时,将与试验申办者联系以确认出版物状态。通过多变量logistic回归评估资金来源对试验未完成的影响,并通过Cox比例风险回归分析对出版时间的影响。控制变量包括干预类型,试验阶段,掩蔽,入组和研究人群。我们分析了659例罕见疾病试验,共计70,305名患者。工业是327个试验(49.6%)的主要资助者,而184个试验(27.9%)的学术机构是主要资助者。有79项针对儿童人群的试验(占12.0%)。总计199个试验(30.2%)被中止。缺乏应计患者(n = 64,32.1%)和信息丰富的终止(n = 41,20.6%)是试验未完成的最常见原因。在已完成的试验中,有306(66.5%)位在2年时尚未发表,而142(31.5%)位在4年时尚未发表。在多变量分析中,与医疗中心资助的试验相比,行业资助的试验被中止的可能性较小(优势比[OR] 2.42; 95%可信区间[CI] 1.34-4.39,P = 0.003)。我们发现资金来源与发布时间之间没有显着关联。共有18,148名患者参加了试验,这些试验在完成后的4年内中止或未发表。我们研究的潜在局限性是,某些针对罕见疾病的干预性试验可能尚未在ClinicalTrials.gov中进行注册,尤其是不需要注册的0期和I期试验。结论在这项研究中,超过半数针对稀有疾病的临床试验在完成后4年就被中止或未发表,导致大量患有稀有疾病的患者受到干预而并未获得有益的发现。需要采取协调一致的措施,以确保患者参与罕见病临床试验可以提高对罕见病的科学知识和治疗水平。不需要注册。结论在这项研究中,超过半数针对稀有疾病的临床试验在完成后4年就被中止或未发表,导致大量患有稀有疾病的患者受到干预而并未获得有益的发现。需要采取协调一致的措施,以确保患者参与罕见病临床试验可以提高对罕见病的科学知识和治疗水平。不需要注册。结论在这项研究中,超过半数针对稀有疾病的临床试验在完成后4年就被中止或未发表,导致大量患有稀有疾病的患者受到干预而并未获得有益的发现。需要采取协调一致的措施,以确保患者参与罕见病临床试验可以提高对罕见病的科学知识和治疗水平。
更新日期:2019-12-03
中文翻译:

研究罕见疾病的试验的非完成和非公开:横断面分析。
背景技术在美国和欧洲,罕见病影响多达6000万人。但是,大多数罕见疾病缺乏有效的治疗方法,因此急需临床研究。我们的目标是确定研究罕见疾病的未完成和未发表的频率。方法和结果我们对随机临床试验进行了横断面分析,该试验研究了由遗传与稀有疾病信息中心数据库定义的稀有疾病,该数据库已于2010年1月1日至2012年12月31日在ClinicalTrials.gov中注册,并已完成或在2014年12月31日停止提供服务。我们的主要结局指标是试验未完成的频率,以及在完成的研究中,试验完成后2年和4年的试验未公开的频率。从注册中心中提取了中止的原因,并根据需要与试验申办者联系以获取更多信息。两名独立研究人员针对PubMed,EMBASE和GoogleScholar中的每个试验进行了出版物检索,从试验完成到发表之间至少需要45个月的时间。当无法确定出版物时,将与试验申办者联系以确认出版物状态。通过多变量logistic回归评估资金来源对试验未完成的影响,并通过Cox比例风险回归分析对出版时间的影响。控制变量包括干预类型,试验阶段,掩蔽,入组和研究人群。我们分析了659例罕见疾病试验,共计70,305名患者。工业是327个试验(49.6%)的主要资助者,而184个试验(27.9%)的学术机构是主要资助者。有79项针对儿童人群的试验(占12.0%)。总计199个试验(30.2%)被中止。缺乏应计患者(n = 64,32.1%)和信息丰富的终止(n = 41,20.6%)是试验未完成的最常见原因。在已完成的试验中,有306(66.5%)位在2年时尚未发表,而142(31.5%)位在4年时尚未发表。在多变量分析中,与医疗中心资助的试验相比,行业资助的试验被中止的可能性较小(优势比[OR] 2.42; 95%可信区间[CI] 1.34-4.39,P = 0.003)。我们发现资金来源与发布时间之间没有显着关联。共有18,148名患者参加了试验,这些试验在完成后的4年内中止或未发表。我们研究的潜在局限性是,某些针对罕见疾病的干预性试验可能尚未在ClinicalTrials.gov中进行注册,尤其是不需要注册的0期和I期试验。结论在这项研究中,超过半数针对稀有疾病的临床试验在完成后4年就被中止或未发表,导致大量患有稀有疾病的患者受到干预而并未获得有益的发现。需要采取协调一致的措施,以确保患者参与罕见病临床试验可以提高对罕见病的科学知识和治疗水平。不需要注册。结论在这项研究中,超过半数针对稀有疾病的临床试验在完成后4年就被中止或未发表,导致大量患有稀有疾病的患者受到干预而并未获得有益的发现。需要采取协调一致的措施,以确保患者参与罕见病临床试验可以提高对罕见病的科学知识和治疗水平。不需要注册。结论在这项研究中,超过半数针对稀有疾病的临床试验在完成后4年就被中止或未发表,导致大量患有稀有疾病的患者受到干预而并未获得有益的发现。需要采取协调一致的措施,以确保患者参与罕见病临床试验可以提高对罕见病的科学知识和治疗水平。



























 京公网安备 11010802027423号
京公网安备 11010802027423号