当前位置:
X-MOL 学术
›
Chem. Bio. Drug Des.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
In silico screening and surface plasma resonance-based verification of programmed death 1-targeted peptides.
Chemical Biology & Drug Design ( IF 3 ) Pub Date : 2019-12-08 , DOI: 10.1111/cbdd.13647 Pengli Zhang 1 , Chengping Li 1 , Xiaoyue Ji 1 , Mingjie Gao 1 , Sifan Lyu 1 , Xiaojun Dai 1 , Jiangfeng Du 1
Chemical Biology & Drug Design ( IF 3 ) Pub Date : 2019-12-08 , DOI: 10.1111/cbdd.13647 Pengli Zhang 1 , Chengping Li 1 , Xiaoyue Ji 1 , Mingjie Gao 1 , Sifan Lyu 1 , Xiaojun Dai 1 , Jiangfeng Du 1
Affiliation
|
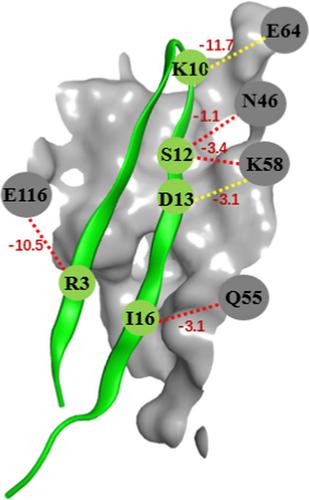
Programmed death 1 (PD-1) is a key immune checkpoint molecule. When it binds to programmed death-ligand 1 (PD-L1), it can negatively regulate the immune response. Therefore, blockade of the PD-1/PD-L1 interaction could unleash the power of immune system. Though successes achieved by anti-PD-1/PD-L1 antibody drugs in clinical for various cancers, many intrinsic limitations of the high molecular weight drugs require alternatives such as peptide drugs and chemical compounds. In this study, we described a novel in silico approach which was used to screen peptides from PDB database and aimed to identify peptides that have potential to bind the PD-L1 binding area of PD-1 molecule. Based on the docking poses, eight peptides were synthesized and measured for their binding abilities by surface plasma resonance technique. The KD values of the synthesized peptides ranged from 10.0 to 133.0 μM. Furthermore, the binding mechanism between PD-1 and the peptides was studied. In conclusion, we established a fast and reliable screening method for peptide discovery, which could be applied for identifying peptide inhibitors of various targets. The synthesized peptides could be served as starting points for designing PD-1 drug for cancer immunotherapy.
中文翻译:

在计算机筛选和基于表面等离子体共振的程序化死亡1靶向肽的验证。
程序性死亡1(PD-1)是关键的免疫检查点分子。当它与程序性死亡配体1(PD-L1)结合时,它可以负面调节免疫反应。因此,PD-1 / PD-L1相互作用的阻断可以释放免疫系统的力量。尽管抗PD-1 / PD-L1抗体药物在各种癌症的临床上取得了成功,但高分子量药物的许多固有局限性要求使用替代药物,例如肽药物和化合物。在这项研究中,我们描述了一种新型的计算机方法,该方法用于从PDB数据库中筛选肽,旨在鉴定具有结合PD-1分子的PD-L1结合区潜力的肽。基于对接姿势,合成了八种肽,并通过表面等离子体共振技术测量了它们的结合能力。合成肽的KD值范围为10.0至133.0μM。此外,研究了PD-1与肽之间的结合机理。总之,我们建立了一种快速,可靠的肽发现筛选方法,可用于鉴定各种靶标的肽抑制剂。合成的多肽可以作为设计PD-1药物用于癌症免疫治疗的起点。
更新日期:2019-12-09
中文翻译:

在计算机筛选和基于表面等离子体共振的程序化死亡1靶向肽的验证。
程序性死亡1(PD-1)是关键的免疫检查点分子。当它与程序性死亡配体1(PD-L1)结合时,它可以负面调节免疫反应。因此,PD-1 / PD-L1相互作用的阻断可以释放免疫系统的力量。尽管抗PD-1 / PD-L1抗体药物在各种癌症的临床上取得了成功,但高分子量药物的许多固有局限性要求使用替代药物,例如肽药物和化合物。在这项研究中,我们描述了一种新型的计算机方法,该方法用于从PDB数据库中筛选肽,旨在鉴定具有结合PD-1分子的PD-L1结合区潜力的肽。基于对接姿势,合成了八种肽,并通过表面等离子体共振技术测量了它们的结合能力。合成肽的KD值范围为10.0至133.0μM。此外,研究了PD-1与肽之间的结合机理。总之,我们建立了一种快速,可靠的肽发现筛选方法,可用于鉴定各种靶标的肽抑制剂。合成的多肽可以作为设计PD-1药物用于癌症免疫治疗的起点。



























 京公网安备 11010802027423号
京公网安备 11010802027423号