Molecular Catalysis ( IF 4.6 ) Pub Date : 2019-11-21 , DOI: 10.1016/j.mcat.2019.110705 Zhen Feng , Yanan Tang , Weiguang Chen , Dong Wei , Yaqiang Ma , Xianqi Dai
|
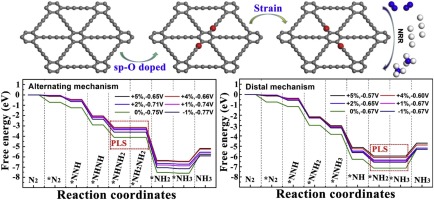
Electrocatalytic nitrogen reduction reaction (NRR) is a sustainable and promising strategy for the conversion of N2 into NH3. However, electrocatalytic NRR is vitally dependent on metal-based catalysts, and it remains a huge challenge in achieving effective NRR on metal-free catalysts. Here we report that O-doped graphdiyne (GDY) as a metal-free NRR electrocatalyst in aqueous solution. O doping in the GDY results in the redistribution of electron density, where the sp-C atom nearest to the O atom provides enhanced capture to N2 molecules. The influence of surface strain on the progression of NRR on O-doped GDY surface has been investigated using the density functional theory (DFT) calculation method. The DFT calculations predict that the potential-limiting steps (PLS) do not vary with the strain, which is *NHNH2→*NH2NH2 for alternating mechanism and *NH2→*NH3 for distal mechanism under the strain range of -1%∼ +5%; the limiting potentials of alternating mechanism is higher than that of distal mechanism; the limiting potentials of two mechanisms decrease with the tensile strain. It can be inferred that the NRR activity increases with a tensile strain of 0%∼ +5%. Our study not only gets deep insights into the catalytic activity of O-doped GDY but also highlights the significance of strain engineering for catalyst design.
中文翻译:

掺杂O的石墨二炔作为无金属的氮还原反应催化剂
电催化氮还原反应(NRR)是将N 2转化为NH 3的可持续且有前途的策略。但是,电催化NRR非常依赖于金属基催化剂,在无金属催化剂上实现有效的NRR仍然是巨大的挑战。在这里,我们报告O掺杂的石墨二炔(GDY)作为水溶液中的无金属NRR电催化剂。GDY中的O掺杂导致电子密度的重新分布,其中最靠近O原子的sp -C原子增强了对N 2的捕获分子。使用密度泛函理论(DFT)计算方法研究了表面应变对N掺杂在O掺杂的GDY表面上NRR进行的影响。DFT计算表明,势能限制步骤(PLS)不会随应变而变化,对于交替机理,这是* NHNH 2 →* NH 2 NH 2,而* NH 2 →* NH 3对于远侧机构,在-1%〜+ 5%的应变范围内;交替机构的极限电位高于远端机构的极限电位。两种机制的极限电势随拉伸应变而降低。可以推断出,NRR活性随着拉伸应变为0%〜+ 5%而增加。我们的研究不仅深入了解了O掺杂GDY的催化活性,而且强调了应变工程对催化剂设计的重要性。



























 京公网安备 11010802027423号
京公网安备 11010802027423号