当前位置:
X-MOL 学术
›
BBA Mol. Cell Res.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Regulation of the autophagic PI3KC3 complex by laforin/malin E3-ubiquitin ligase, two proteins involved in Lafora disease.
Biochimica et Biophysica Acta (BBA) - Molecular Cell Research ( IF 5.1 ) Pub Date : 2019-11-21 , DOI: 10.1016/j.bbamcr.2019.118613 Pablo Sanchez-Martin 1 , Marcos Lahuerta 1 , Rosa Viana 1 , Erwin Knecht 2 , Pascual Sanz 3
Biochimica et Biophysica Acta (BBA) - Molecular Cell Research ( IF 5.1 ) Pub Date : 2019-11-21 , DOI: 10.1016/j.bbamcr.2019.118613 Pablo Sanchez-Martin 1 , Marcos Lahuerta 1 , Rosa Viana 1 , Erwin Knecht 2 , Pascual Sanz 3
Affiliation
|
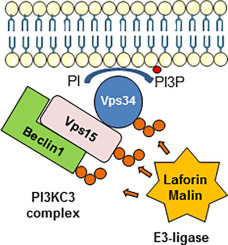
Lafora progressive myoclonus epilepsy is a fatal rare neurodegenerative disorder characterized by the accumulation of insoluble abnormal glycogen deposits in the brain and peripheral tissues. Mutations in at least two genes are responsible for the disease: EPM2A, encoding the glucan phosphatase laforin, and EPM2B, encoding the RING-type E3-ubiquitin ligase malin. Both laforin and malin form a functional complex in which laforin recruits the substrates to be ubiquitinated by malin. We and others have described that, in cellular and animal models of this disease, there is an autophagy impairment which leads to the accumulation of dysfunctional mitochondria. In addition, we established that the autophagic defect occurred at the initial steps of autophagosome formation. In this work, we present evidence that in cellular models of the disease there is a decrease in the amount of phosphatidylinositol-3P. This is probably due to defective regulation of the autophagic PI3KC3 complex, in the absence of a functional laforin/malin complex. In fact, we demonstrate that the laforin/malin complex interacts physically and co-localizes intracellularly with core components of the PI3KC3 complex (Beclin1, Vps34 and Vps15), and that this interaction is specific and results in the polyubiquitination of these proteins. In addition, the laforin/malin complex is also able to polyubiquitinate ATG14L and UVRAG. Finally, we show that overexpression of the laforin/malin complex increases PI3KC3 activity. All these results suggest a new role of the laforin/malin complex in the activation of autophagy via regulation of the PI3KC3 complex and explain the defect in autophagy described in Lafora disease.
中文翻译:

Laforin / malin E3-泛素连接酶(参与Lafora疾病的两种蛋白质)对自噬PI3KC3复合物的调节。
拉福拉进行性肌阵挛性癫痫是一种致命的罕见神经退行性疾病,其特征是在大脑和周围组织中积累了不可溶的异常糖原沉积物。至少两个基因的突变是造成该疾病的原因:编码葡聚糖磷酸酶laforin的EPM2A和编码RING型E3-泛素连接酶马林蛋白的EPM2B。laforin和malin均形成功能复合物,其中laforin募集底物以被malin泛素化。我们和其他人描述,在这种疾病的细胞和动物模型中,存在自噬损伤,导致功能障碍线粒体积聚。另外,我们确定自噬缺陷发生在自噬体形成的初始步骤。在这项工作中,我们提供的证据表明,在该疾病的细胞模型中,磷脂酰肌醇3P的数量有所减少。这可能是由于在缺乏功能性laforin / malin复合物的情况下对自噬PI3KC3复合物调控的缺陷。实际上,我们证明了laforin / malin复合物与PI3KC3复合物的核心成分(Beclin1,Vps34和Vps15)发生物理相互作用并在细胞内共定位,并且这种相互作用是特异性的,并导致这些蛋白的多泛素化。另外,laforin / malin复合物还能够将ATG14L和UVRAG泛素化。最后,我们显示了laforin / malin复合物的过表达增加了PI3KC3的活性。
更新日期:2019-11-21
中文翻译:

Laforin / malin E3-泛素连接酶(参与Lafora疾病的两种蛋白质)对自噬PI3KC3复合物的调节。
拉福拉进行性肌阵挛性癫痫是一种致命的罕见神经退行性疾病,其特征是在大脑和周围组织中积累了不可溶的异常糖原沉积物。至少两个基因的突变是造成该疾病的原因:编码葡聚糖磷酸酶laforin的EPM2A和编码RING型E3-泛素连接酶马林蛋白的EPM2B。laforin和malin均形成功能复合物,其中laforin募集底物以被malin泛素化。我们和其他人描述,在这种疾病的细胞和动物模型中,存在自噬损伤,导致功能障碍线粒体积聚。另外,我们确定自噬缺陷发生在自噬体形成的初始步骤。在这项工作中,我们提供的证据表明,在该疾病的细胞模型中,磷脂酰肌醇3P的数量有所减少。这可能是由于在缺乏功能性laforin / malin复合物的情况下对自噬PI3KC3复合物调控的缺陷。实际上,我们证明了laforin / malin复合物与PI3KC3复合物的核心成分(Beclin1,Vps34和Vps15)发生物理相互作用并在细胞内共定位,并且这种相互作用是特异性的,并导致这些蛋白的多泛素化。另外,laforin / malin复合物还能够将ATG14L和UVRAG泛素化。最后,我们显示了laforin / malin复合物的过表达增加了PI3KC3的活性。

























 京公网安备 11010802027423号
京公网安备 11010802027423号