当前位置:
X-MOL 学术
›
Chin. J. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
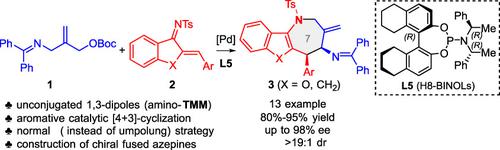
Palladium‐Catalyzed Asymmetric [4+3]‐Cyclization Reaction of Fused 1‐Azadienes with Amino‐trimethylenemethanes: Highly Stereoselective Construction of Chiral Fused Azepines
Chinese Journal of Chemistry ( IF 5.4 ) Pub Date : 2019-12-24 , DOI: 10.1002/cjoc.201900430 Prathibha Kumari 1 , Weiwei Liu 1 , Cheng‐Jie Wang 1 , Jun Dai 1 , Mei‐Xin Wang 1 , Qi‐Qiong Yang 1 , Yu‐Hua Deng 1 , Zhihui Shao 1
Chinese Journal of Chemistry ( IF 5.4 ) Pub Date : 2019-12-24 , DOI: 10.1002/cjoc.201900430 Prathibha Kumari 1 , Weiwei Liu 1 , Cheng‐Jie Wang 1 , Jun Dai 1 , Mei‐Xin Wang 1 , Qi‐Qiong Yang 1 , Yu‐Hua Deng 1 , Zhihui Shao 1
Affiliation
|
A Pd‐catalyzed asymmetric aromative [4+3]‐cyclization reaction of amino‐trimethylenemethanes (TMM, 1,3‐dipoles) with fused 1‐azadienes has been developed. This method enables access to the synthetically importance and biologically active benzofuran fused azepines and indeno‐azepines in excellent efficiency and stereoselectivity (up to 95% yield, 99% ee, >19 : 1 dr).
中文翻译:

钯催化的1-氮杂二氮杂与氨基三亚甲基甲烷的不对称[4 + 3]-环化反应:手性熔融氮杂环丁烷的高度立体选择性构建
已经开发了Pd催化的氨基三亚甲基甲烷(TMM,1,3-偶极子)与稠合的1-氮杂二烯的不对称芳香[4 + 3]环化反应。这种方法能够以优异的效率和立体选择性(高达95%的收率,99%ee,> 19:1 dr)获得具有合成重要性的,具有生物活性的苯并呋喃稠合的氮杂品和茚并-氮杂品。
更新日期:2019-12-25
中文翻译:

钯催化的1-氮杂二氮杂与氨基三亚甲基甲烷的不对称[4 + 3]-环化反应:手性熔融氮杂环丁烷的高度立体选择性构建
已经开发了Pd催化的氨基三亚甲基甲烷(TMM,1,3-偶极子)与稠合的1-氮杂二烯的不对称芳香[4 + 3]环化反应。这种方法能够以优异的效率和立体选择性(高达95%的收率,99%ee,> 19:1 dr)获得具有合成重要性的,具有生物活性的苯并呋喃稠合的氮杂品和茚并-氮杂品。


























 京公网安备 11010802027423号
京公网安备 11010802027423号