当前位置:
X-MOL 学术
›
J. Comput. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Membrane lipids are both the substrates and a mechanistically responsive environment of TMEM16 scramblase proteins
Journal of Computational Chemistry ( IF 3 ) Pub Date : 2019-11-21 , DOI: 10.1002/jcc.26105 George Khelashvili 1, 2 , Xiaolu Cheng 1 , Maria E Falzone 3 , Milka Doktorova 4 , Alessio Accardi 1, 3, 5 , Harel Weinstein 1, 2
Journal of Computational Chemistry ( IF 3 ) Pub Date : 2019-11-21 , DOI: 10.1002/jcc.26105 George Khelashvili 1, 2 , Xiaolu Cheng 1 , Maria E Falzone 3 , Milka Doktorova 4 , Alessio Accardi 1, 3, 5 , Harel Weinstein 1, 2
Affiliation
|
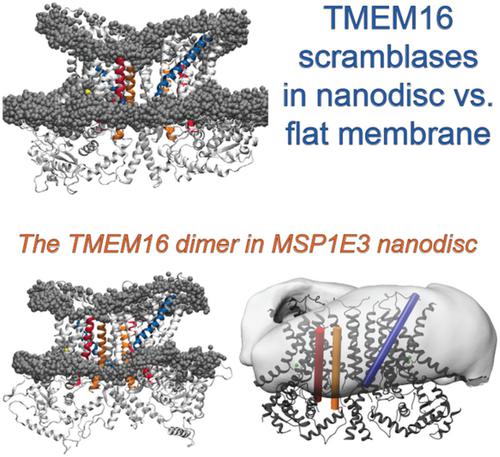
Recent discoveries about functional mechanisms of proteins in the TMEM16 family of phospholipid scramblases have illuminated the dual role of the membrane as both the substrate and a mechanistically responsive environment in the wide range of physiological processes and genetic disorders in which they are implicated. This is highlighted in the review of recent findings from our collaborative investigations of molecular mechanisms of TMEM16 scramblases that emerged from iterative functional, structural, and computational experimentation. In the context of this review, we present new MD simulations and trajectory analyses motivated by the fact that new structural information about the TMEM16 scramblases is emerging from cryo‐EM determinations in lipid nanodiscs. Because the functional environment of these proteins in in vivo and in in vitro is closer to flat membranes, we studied comparatively the responses of the membrane to the TMEM16 proteins in flat membranes and nanodiscs. We find that bilayer shapes in the nanodiscs are very different from those observed in the flat membrane systems, but the function‐related slanting of the membrane observed at the nhTMEM16 boundary with the protein is similar in the nanodiscs and in the flat bilayers. This changes, however, in the bilayer composed of longer‐tail lipids, which is thicker near the phospholipid translocation pathway, which may reflect an enhanced tendency of the long tails to penetrate the pathway and create, as shown previously, a nonconductive environment. These findings support the correspondence between the mechanistic involvement of the lipid environment in the flat membranes, and the nanodiscs. © 2019 Wiley Periodicals, Inc.
中文翻译:

膜脂既是 TMEM16 扰乱酶蛋白的底物又是机械响应环境
关于磷脂混杂酶 TMEM16 家族中蛋白质功能机制的最新发现阐明了膜在广泛的生理过程和遗传性疾病中作为底物和机械响应环境的双重作用。我们在迭代功能、结构和计算实验中对 TMEM16 扰乱酶的分子机制进行的合作研究的最新发现的回顾中强调了这一点。在这篇综述的背景下,我们提出了新的 MD 模拟和轨迹分析,其动机是通过脂质纳米盘中的冷冻电镜测定得出有关 TMEM16 扰乱酶的新结构信息。由于这些蛋白在体内和体外的功能环境更接近于平板膜,因此我们比较研究了平板膜和纳米圆盘中膜对TMEM16蛋白的响应。我们发现纳米盘中的双层形状与在平坦膜系统中观察到的双层形状非常不同,但在 nhTMEM16 与蛋白质边界处观察到的膜的功能相关倾斜在纳米盘和平坦双层中是相似的。然而,这种变化在由长尾脂质组成的双层中发生了变化,该双层在磷脂易位途径附近更厚,这可能反映了长尾穿透该途径并产生非导电环境的趋势增强,如前所述。这些发现支持了平坦膜中脂质环境的机械参与与纳米圆盘之间的对应关系。© 2019 Wiley 期刊公司。
更新日期:2019-11-21
中文翻译:

膜脂既是 TMEM16 扰乱酶蛋白的底物又是机械响应环境
关于磷脂混杂酶 TMEM16 家族中蛋白质功能机制的最新发现阐明了膜在广泛的生理过程和遗传性疾病中作为底物和机械响应环境的双重作用。我们在迭代功能、结构和计算实验中对 TMEM16 扰乱酶的分子机制进行的合作研究的最新发现的回顾中强调了这一点。在这篇综述的背景下,我们提出了新的 MD 模拟和轨迹分析,其动机是通过脂质纳米盘中的冷冻电镜测定得出有关 TMEM16 扰乱酶的新结构信息。由于这些蛋白在体内和体外的功能环境更接近于平板膜,因此我们比较研究了平板膜和纳米圆盘中膜对TMEM16蛋白的响应。我们发现纳米盘中的双层形状与在平坦膜系统中观察到的双层形状非常不同,但在 nhTMEM16 与蛋白质边界处观察到的膜的功能相关倾斜在纳米盘和平坦双层中是相似的。然而,这种变化在由长尾脂质组成的双层中发生了变化,该双层在磷脂易位途径附近更厚,这可能反映了长尾穿透该途径并产生非导电环境的趋势增强,如前所述。这些发现支持了平坦膜中脂质环境的机械参与与纳米圆盘之间的对应关系。© 2019 Wiley 期刊公司。


























 京公网安备 11010802027423号
京公网安备 11010802027423号