当前位置:
X-MOL 学术
›
Drug Test. Anal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Detection of 5α-reductase inhibitors by UPLC-MS/MS: Application to the definition of the excretion profile of dutasteride in urine.
Drug Testing and Analysis ( IF 2.9 ) Pub Date : 2019-11-20 , DOI: 10.1002/dta.2702 Monica Mazzarino 1 , Lorenzo Martellone 1 , Fabio Comunità 1 , Xavier de la Torre 1 , Francesco Molaioni 1 , Francesco Botrè 1, 2
Drug Testing and Analysis ( IF 2.9 ) Pub Date : 2019-11-20 , DOI: 10.1002/dta.2702 Monica Mazzarino 1 , Lorenzo Martellone 1 , Fabio Comunità 1 , Xavier de la Torre 1 , Francesco Molaioni 1 , Francesco Botrè 1, 2
Affiliation
|
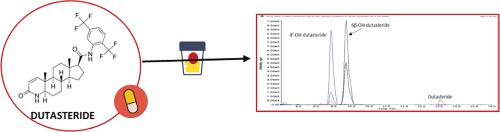
An analytical procedure based on ultra‐performance liquid chromatography‐mass spectrometry was developed to screen and to confirm dutasteride and its metabolites in human urine. Sample preparation included an enzymatic hydrolysis followed by solid‐phase extraction using the strong cation exchange cartridges OASIS® MCX. The chromatographic separation was carried out on C18 column, employing as mobile phases ultra purified water and acetonitrile, both containing 0.1% formic acid. Detection was achieved using a triple quadrupole as a mass spectrometric analyzer, with positive ion electrospray ionization and multiple reaction monitoring as acquisition mode. The analytical procedure developed was validated according to ISO 17025 and World Anti‐Doping Agency guidelines. The extraction efficiency was estimated to be greater than 75% for both dutasteride and its hydroxylated metabolites. Detection capability was determined in the range of 0.1–0.4 ng/mL. Specificity and repeatability of the relative retention times (CV% < 0.5) and of the relative abundances of the characteristic ion transitions selected (CV% < 10) were confirmed to be fit for purpose to ensure the unambiguous identification of dutasteride and its metabolites in human urine. The developed method was used to characterize the urinary excretion profile of dutasteride after both chronic and acute administration of therapeutic doses. After chronic administration, dutasteride and its hydroxylated metabolites were easily detected and confirmed. After acute administration, instead, only the two hydroxylated metabolites were detected for 3–4 days.
中文翻译:

通过UPLC-MS / MS检测5α-还原酶抑制剂:在定义度他雄胺在尿中的排泄特性方面的应用。
开发了一种基于超高效液相色谱-质谱法的分析程序,以筛选和确认人尿中的度他雄胺及其代谢产物。样品制备包括酶水解,接着用强阳离子交换盒OASIS通过固相萃取®MCX。色谱分离在C18柱上进行,采用均含有0.1%甲酸的超纯水和乙腈作为流动相。使用三重四极杆作为质谱分析仪,以正离子电喷雾电离和多反应监测作为采集模式,实现了检测。所开发的分析程序已根据ISO 17025和世界反兴奋剂机构指南进行了验证。据估计,度他雄胺及其羟基化代谢物的提取效率均高于75%。确定的检测能力在0.1–0.4 ng / mL的范围内。相对保留时间(CV%<0.5)和所选特征离子跃迁的相对丰度(CV%< 10)被证实适合用于确保人尿中度他雄胺及其代谢物的明确鉴定。长期和急性给予治疗剂量后,所开发的方法用于表征度他雄胺的尿排泄特征。长期给药后,很容易检测和确认度他雄胺及其羟基化代谢产物。急性给药后,在3-4天中仅检测到两种羟基化代谢物。
更新日期:2019-11-20
中文翻译:

通过UPLC-MS / MS检测5α-还原酶抑制剂:在定义度他雄胺在尿中的排泄特性方面的应用。
开发了一种基于超高效液相色谱-质谱法的分析程序,以筛选和确认人尿中的度他雄胺及其代谢产物。样品制备包括酶水解,接着用强阳离子交换盒OASIS通过固相萃取®MCX。色谱分离在C18柱上进行,采用均含有0.1%甲酸的超纯水和乙腈作为流动相。使用三重四极杆作为质谱分析仪,以正离子电喷雾电离和多反应监测作为采集模式,实现了检测。所开发的分析程序已根据ISO 17025和世界反兴奋剂机构指南进行了验证。据估计,度他雄胺及其羟基化代谢物的提取效率均高于75%。确定的检测能力在0.1–0.4 ng / mL的范围内。相对保留时间(CV%<0.5)和所选特征离子跃迁的相对丰度(CV%< 10)被证实适合用于确保人尿中度他雄胺及其代谢物的明确鉴定。长期和急性给予治疗剂量后,所开发的方法用于表征度他雄胺的尿排泄特征。长期给药后,很容易检测和确认度他雄胺及其羟基化代谢产物。急性给药后,在3-4天中仅检测到两种羟基化代谢物。



























 京公网安备 11010802027423号
京公网安备 11010802027423号