当前位置:
X-MOL 学术
›
Biomater. Sci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Multifunctional hybrid nanoconstructs facilitate intracellular localization of doxorubicin and genistein to enhance apoptotic and anti-angiogenic efficacy in breast adenocarcinoma.
Biomaterials Science ( IF 6.6 ) Pub Date : 2019-11-21 , DOI: 10.1039/c9bm01246j Ravi Prakash Shukla 1 , Jayant Dewangan , Sandeep Urandur , Venkatesh Teja Banala , Monika Diwedi , Shweta Sharma , Sristi Agrawal , Srikanta Kumar Rath , Ritu Trivedi , Prabhat Ranjan Mishra
Biomaterials Science ( IF 6.6 ) Pub Date : 2019-11-21 , DOI: 10.1039/c9bm01246j Ravi Prakash Shukla 1 , Jayant Dewangan , Sandeep Urandur , Venkatesh Teja Banala , Monika Diwedi , Shweta Sharma , Sristi Agrawal , Srikanta Kumar Rath , Ritu Trivedi , Prabhat Ranjan Mishra
Affiliation
|
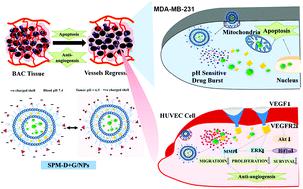
The progressive development of tumors leading to angiogenesis marks the advancement of cancer which requires specific targeted treatment preferably with combination chemotherapy. However, there is still a long way to go to develop an efficient delivery system that could overcome the tumor microenvironment to achieve efficient delivery. Therefore, we have developed spermine (SPM) tethered lipo-polymeric hybrid nanoconstructs with cell surface heparan sulfate proteoglycan (HSPG) specificity for higher intracellular localization and pH dependent charge reversal in the tumor microenvironment (below pH 5.8) to facilitate Doxorubicin (Dox) and Genistein (Gen) release in a synergistic combination. We have observed the specific uptake of SPM anchored hybrid nanoconstructs by receptor-mediated endocytosis in human breast cancer cells (MDA-MB-231) through the HSPG receptor. The SPM-D + G/NPs induced a higher rate of apoptosis in MDA-MB-231 cells via disruption of the mitochondrial membrane potential and also exhibited a stronger anti-angiogenic effect governing the inhibition of VEGF pathway modulation, proliferation, invasion and migration of HUVECs in in vitro and in vivo Balb/c mouse models. The involvement of Akt/Hif1α/VEGF dependent signal cascading and its down-regulation with a pro-apoptotic drug Dox and an anti-angiogenic agent Gen was evident as demonstrated by an in silico docking study and subsequently proven by RT-PCR and western blotting. Altogether this study highlights the potential role of SPM in targeting HSPG receptors and synergistic delivery of Dox and Gen as a promising strategy to effectively inhibit BAC progression and these findings could open a new window to deliver combinations of chemotherapeutic agents along with anti-angiogenic ligands using hybrid nanoparticles.
中文翻译:

多功能杂合纳米结构促进阿霉素和染料木黄酮在细胞内的定位,从而增强乳腺癌和腺癌的凋亡和抗血管生成功效。
导致血管生成的肿瘤的逐步发展标志着癌症的发展,其需要特定的靶向治疗,优选联合化疗。然而,开发有效的递送系统还有很长的路要走,该系统可以克服肿瘤的微环境以实现有效的递送。因此,我们开发了具有细胞表面硫酸乙酰肝素蛋白聚糖(HSPG)特异性的精胺(SPM)拴系的脂聚合物杂化纳米结构,可在肿瘤微环境(pH 5.8以下)中实现更高的细胞内定位和pH依赖性电荷逆转,以促进阿霉素(Dox)和Genistein(Gen)以协同作用释放。我们已经观察到人类乳腺癌细胞(MDA-MB-231)中通过HSPG受体通过受体介导的内吞作用特异性吸收了SPM锚定的杂合纳米结构。SPM-D + G / NPs诱导MDA-MB-231细胞凋亡率更高通过破坏线粒体膜电位,还表现出更强的抗血管生成作用,可在体外和体内Balb / c小鼠模型中控制VEGF信号通路的调节,抑制HUVEC的增殖,侵袭和迁移。如in silico所证明的,Akt /Hif1α/ VEGF依赖性信号级联的参与及其对促凋亡药物Dox和抗血管生成剂Gen的下调是明显的。对接研究,随后通过RT-PCR和Western blotting进行了验证。总而言之,这项研究强调了SPM在靶向HSPG受体以及Dox和Gen协同递送方面的潜在作用,这是有效抑制BAC进展的有前途的策略,这些发现可能为使用化学治疗剂与抗血管生成配体的组合提供新的窗口。杂化纳米粒子。
更新日期:2019-11-21
中文翻译:

多功能杂合纳米结构促进阿霉素和染料木黄酮在细胞内的定位,从而增强乳腺癌和腺癌的凋亡和抗血管生成功效。
导致血管生成的肿瘤的逐步发展标志着癌症的发展,其需要特定的靶向治疗,优选联合化疗。然而,开发有效的递送系统还有很长的路要走,该系统可以克服肿瘤的微环境以实现有效的递送。因此,我们开发了具有细胞表面硫酸乙酰肝素蛋白聚糖(HSPG)特异性的精胺(SPM)拴系的脂聚合物杂化纳米结构,可在肿瘤微环境(pH 5.8以下)中实现更高的细胞内定位和pH依赖性电荷逆转,以促进阿霉素(Dox)和Genistein(Gen)以协同作用释放。我们已经观察到人类乳腺癌细胞(MDA-MB-231)中通过HSPG受体通过受体介导的内吞作用特异性吸收了SPM锚定的杂合纳米结构。SPM-D + G / NPs诱导MDA-MB-231细胞凋亡率更高通过破坏线粒体膜电位,还表现出更强的抗血管生成作用,可在体外和体内Balb / c小鼠模型中控制VEGF信号通路的调节,抑制HUVEC的增殖,侵袭和迁移。如in silico所证明的,Akt /Hif1α/ VEGF依赖性信号级联的参与及其对促凋亡药物Dox和抗血管生成剂Gen的下调是明显的。对接研究,随后通过RT-PCR和Western blotting进行了验证。总而言之,这项研究强调了SPM在靶向HSPG受体以及Dox和Gen协同递送方面的潜在作用,这是有效抑制BAC进展的有前途的策略,这些发现可能为使用化学治疗剂与抗血管生成配体的组合提供新的窗口。杂化纳米粒子。



























 京公网安备 11010802027423号
京公网安备 11010802027423号