当前位置:
X-MOL 学术
›
Int. J. Quantum Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Theoretical study of the desorption of neutral and ionic alkali metal atoms from the excited Li+(H2O)n = 1‐4 and Na+(H2O)n = 1‐4 cluster models: Electronic excitation charge transfer
International Journal of Quantum Chemistry ( IF 2.2 ) Pub Date : 2019-11-19 , DOI: 10.1002/qua.26104 Hossein Farrokhpour 1 , Samaneh Khoshkhou 1
International Journal of Quantum Chemistry ( IF 2.2 ) Pub Date : 2019-11-19 , DOI: 10.1002/qua.26104 Hossein Farrokhpour 1 , Samaneh Khoshkhou 1
Affiliation
|
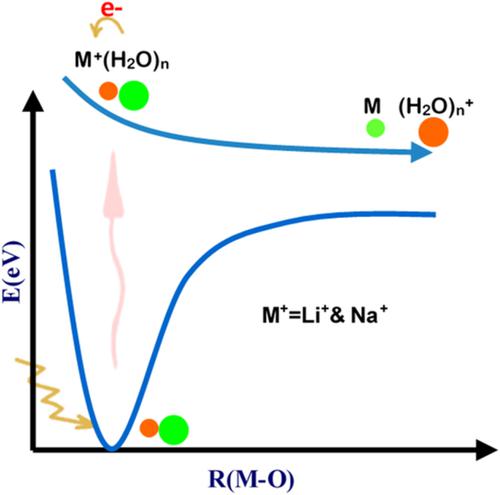
In this work, the potential energy curves of several low‐lying excited states of M+(H2O)n = 1‐4 (M = Li and Na) clusters with one M─O bond, related to the stretching of their M─O bond, were calculated in the gas phase. The time‐dependent density functional theory and direct‐symmetry‐adapted cluster‐configuration interaction were used in this study separately. Theoretical calculations showed that the charge transfer occurred between M+ and (H2O)n in the excited clusters so that the neutral metal atom was obtained at the dissociation limit of the potential curves. The excited potential curves of clusters were also calculated in the presence of the electrostatic field of water (EFW), and it was found that the charge transfer was blocked in the presence of EFW. The effect of the size of the (H2O)n cluster on the shape of the excited potential curves was investigated to observe how the M─O bond was affected in the excited states depending on the (H2O)n size. It was found that the increase in the size of the (H2O)n cluster increased the number of bonding excited potential curves. The difference between the electron density of the excited and ground electronic states was calculated to see how the charge transfer was affected by the size of the (H2O)n cluster.
中文翻译:

Li +(H2O)n = 1-4和Na +(H2O)n = 1-4团簇模型解吸中性和离子性碱金属原子的理论研究:电子激发电荷转移
在这项工作中,具有一个M- O键的M +(H 2 O)n = 1-4(M = Li和Na)团簇的几个低激发态的势能曲线与其M的拉伸有关─O键,是在气相中计算的。本研究分别使用了基于时间的密度泛函理论和直接对称适应的簇-构型相互作用。理论计算表明,电荷转移发生在M +和(H 2 O)n之间激发团簇中的碳原子,从而在电势曲线的解离极限处获得中性金属原子。还在水的静电场(EFW)存在下计算了团簇的激发电势曲线,发现在EFW存在下电荷转移受到了阻碍。研究了(H 2 O)n团簇的大小对激发电势曲线形状的影响,以观察在(H 2 O)n大小的激发态下,M- O键是如何受到影响的。发现(H 2 O)n的尺寸增加团簇增加了键合激发电势曲线的数量。计算激发态和基态电子态的电子密度之间的差异,以了解电荷转移如何受到(H 2 O)n团簇尺寸的影响。
更新日期:2020-01-07
中文翻译:

Li +(H2O)n = 1-4和Na +(H2O)n = 1-4团簇模型解吸中性和离子性碱金属原子的理论研究:电子激发电荷转移
在这项工作中,具有一个M- O键的M +(H 2 O)n = 1-4(M = Li和Na)团簇的几个低激发态的势能曲线与其M的拉伸有关─O键,是在气相中计算的。本研究分别使用了基于时间的密度泛函理论和直接对称适应的簇-构型相互作用。理论计算表明,电荷转移发生在M +和(H 2 O)n之间激发团簇中的碳原子,从而在电势曲线的解离极限处获得中性金属原子。还在水的静电场(EFW)存在下计算了团簇的激发电势曲线,发现在EFW存在下电荷转移受到了阻碍。研究了(H 2 O)n团簇的大小对激发电势曲线形状的影响,以观察在(H 2 O)n大小的激发态下,M- O键是如何受到影响的。发现(H 2 O)n的尺寸增加团簇增加了键合激发电势曲线的数量。计算激发态和基态电子态的电子密度之间的差异,以了解电荷转移如何受到(H 2 O)n团簇尺寸的影响。

























 京公网安备 11010802027423号
京公网安备 11010802027423号