当前位置:
X-MOL 学术
›
Green Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Natural deep eutectic solvents as an efficient and reusable active system for the Nazarov cyclization
Green Chemistry ( IF 9.8 ) Pub Date : 2019-11-19 , DOI: 10.1039/c9gc03465j Stefano Nejrotti 1, 2, 3, 4 , Marta Iannicelli 1, 2, 3, 4 , Salwa Simona Jamil 1, 2, 3, 4 , Davide Arnodo 1, 2, 3, 4 , Marco Blangetti 1, 2, 3, 4 , Cristina Prandi 1, 2, 3, 4
Green Chemistry ( IF 9.8 ) Pub Date : 2019-11-19 , DOI: 10.1039/c9gc03465j Stefano Nejrotti 1, 2, 3, 4 , Marta Iannicelli 1, 2, 3, 4 , Salwa Simona Jamil 1, 2, 3, 4 , Davide Arnodo 1, 2, 3, 4 , Marco Blangetti 1, 2, 3, 4 , Cristina Prandi 1, 2, 3, 4
Affiliation
|
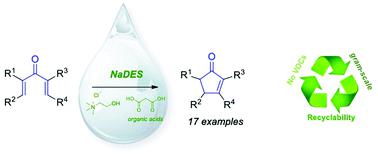
Natural deep eutectic solvents have emerged as alternative non-toxic, non-aqueous solvents for an increasing number of synthetic transformations. Remarkably, in some cases one (or more) components of the NaDES plays an active role in the reaction mechanism and directly participates as either a catalyst or a reagent in the reaction. In this paper, we tested several NaDESs in which one of the components is a carboxylic acid as a medium to perform the Nazarov cyclization of divinyl ketones to obtain cyclopentenones, a widespread motif in natural compounds. The reaction conditions were optimized and the scope was investigated on C-, O- and N-derived compounds. To assess the full sustainability of the proposed approach, the recyclability and scalability of the process were investigated, thus proving that multi-gram preparations are possible with complete recycling of the medium.
中文翻译:

天然深共晶溶剂可作为Nazarov环化反应的有效和可重复使用的活性系统
天然的深共熔溶剂已成为替代的无毒,无水溶剂,可用于越来越多的合成转化中。值得注意的是,在某些情况下,NaDES的一种(或多种)组分在反应机理中起积极作用,并直接作为催化剂或试剂参与反应。在本文中,我们测试了几种NaDES,其中的一种组分是羧酸作为介质,以进行二乙烯基酮的Nazarov环化反应,获得环戊烯酮,这是天然化合物中普遍存在的基序。优化了反应条件,研究了C-,O-和N的范围。衍生的化合物。为了评估所提出方法的完全可持续性,对工艺的可回收性和可扩展性进行了研究,从而证明在完全回收介质的情况下,可以进行多克制剂的制备。
更新日期:2019-11-19
中文翻译:

天然深共晶溶剂可作为Nazarov环化反应的有效和可重复使用的活性系统
天然的深共熔溶剂已成为替代的无毒,无水溶剂,可用于越来越多的合成转化中。值得注意的是,在某些情况下,NaDES的一种(或多种)组分在反应机理中起积极作用,并直接作为催化剂或试剂参与反应。在本文中,我们测试了几种NaDES,其中的一种组分是羧酸作为介质,以进行二乙烯基酮的Nazarov环化反应,获得环戊烯酮,这是天然化合物中普遍存在的基序。优化了反应条件,研究了C-,O-和N的范围。衍生的化合物。为了评估所提出方法的完全可持续性,对工艺的可回收性和可扩展性进行了研究,从而证明在完全回收介质的情况下,可以进行多克制剂的制备。


























 京公网安备 11010802027423号
京公网安备 11010802027423号