当前位置:
X-MOL 学术
›
Chem. Sci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Exploiting the versatile alkyne-based chemistry for expanding the applications of a stable triphenylmethyl organic radical on surfaces
Chemical Science ( IF 8.4 ) Pub Date : 2019-11-20 , DOI: 10.1039/c9sc04499j J Alejandro de Sousa 1, 2 , Francesc Bejarano 1 , Diego Gutiérrez 1 , Yann R Leroux 3 , Ewa Malgorzata Nowik-Boltyk 4 , Tobias Junghoefer 4 , Erika Giangrisostomi 5 , Ruslan Ovsyannikov 5 , Maria Benedetta Casu 4 , Jaume Veciana 1 , Marta Mas-Torrent 1 , Bruno Fabre 3 , Concepció Rovira 1 , Núria Crivillers 1
Chemical Science ( IF 8.4 ) Pub Date : 2019-11-20 , DOI: 10.1039/c9sc04499j J Alejandro de Sousa 1, 2 , Francesc Bejarano 1 , Diego Gutiérrez 1 , Yann R Leroux 3 , Ewa Malgorzata Nowik-Boltyk 4 , Tobias Junghoefer 4 , Erika Giangrisostomi 5 , Ruslan Ovsyannikov 5 , Maria Benedetta Casu 4 , Jaume Veciana 1 , Marta Mas-Torrent 1 , Bruno Fabre 3 , Concepció Rovira 1 , Núria Crivillers 1
Affiliation
|
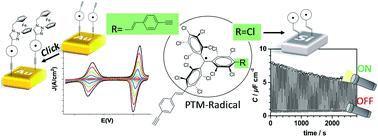
The incorporation of terminal alkynes into the chemical structure of persistent organic perchlorotriphenylmethyl (PTM) radicals provides new chemical tools to expand their potential applications. In this work, this is demonstrated by the chemical functionalization of two types of substrates, hydrogenated SiO2-free silicon (Si–H) and gold, and, by exploiting the click chemistry, scarcely used with organic radicals, to synthesise multifunctional systems. On one hand, the one-step functionalization of Si–H allows a light-triggered capacitance switch to be successfully achieved under electrochemical conditions. On the other hand, the click reaction between the alkyne-terminated PTM radical and a ferrocene azide derivative, used here as a model azide system, leads to a multistate electrochemical switch. The successful post-surface modification makes the self-assembled monolayers reported here an appealing platform to synthesise multifunctional systems grafted on surfaces.
中文翻译:

利用基于炔烃的通用化学方法扩展稳定的三苯甲基有机自由基在表面上的应用
将末端炔烃结合到持久性有机全氯三苯甲基 (PTM) 自由基的化学结构中,为扩展其潜在应用提供了新的化学工具。在这项工作中,这通过两种基材的化学功能化来证明,氢化 SiO 2-游离硅(Si-H)和金,并通过利用几乎不与有机自由基一起使用的点击化学来合成多功能系统。一方面,Si-H 的一步功能化允许在电化学条件下成功实现光触发电容开关。另一方面,炔烃封端的 PTM 自由基和二茂铁叠氮化物衍生物之间的点击反应(此处用作模型叠氮化物系统)导致多态电化学开关。成功的后表面改性使这里报道的自组装单分子层成为合成接枝在表面上的多功能系统的有吸引力的平台。
更新日期:2019-11-20
中文翻译:

利用基于炔烃的通用化学方法扩展稳定的三苯甲基有机自由基在表面上的应用
将末端炔烃结合到持久性有机全氯三苯甲基 (PTM) 自由基的化学结构中,为扩展其潜在应用提供了新的化学工具。在这项工作中,这通过两种基材的化学功能化来证明,氢化 SiO 2-游离硅(Si-H)和金,并通过利用几乎不与有机自由基一起使用的点击化学来合成多功能系统。一方面,Si-H 的一步功能化允许在电化学条件下成功实现光触发电容开关。另一方面,炔烃封端的 PTM 自由基和二茂铁叠氮化物衍生物之间的点击反应(此处用作模型叠氮化物系统)导致多态电化学开关。成功的后表面改性使这里报道的自组装单分子层成为合成接枝在表面上的多功能系统的有吸引力的平台。


























 京公网安备 11010802027423号
京公网安备 11010802027423号