当前位置:
X-MOL 学术
›
Angew. Chem. Int. Ed.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Development and Elucidation of a Pd-Based Cyclization-Oxygenation Sequence for Natural Product Synthesis.
Angewandte Chemie International Edition ( IF 16.6 ) Pub Date : 2020-01-08 , DOI: 10.1002/anie.201913730 Heng Yi 1 , Pengfei Hu 1 , Scott A Snyder 1
Angewandte Chemie International Edition ( IF 16.6 ) Pub Date : 2020-01-08 , DOI: 10.1002/anie.201913730 Heng Yi 1 , Pengfei Hu 1 , Scott A Snyder 1
Affiliation
|
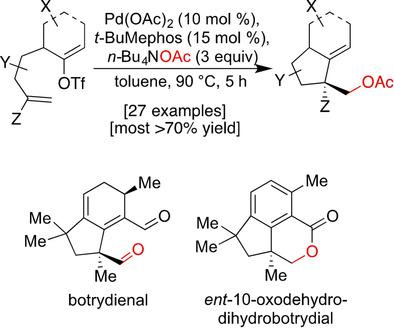
Pd-catalyzed sequences involving oxidative addition, cyclization, and termination through intermolecular nucleophile capture have tremendous utility. Indeed, they can generate a plethora of different polycyclic structures possessing a diverse range of functionality. However, one area of deficiency for Pd0 /PdII variants is the ability to conclude them with oxygen-based species. Inspired by the recent discovery of one such reaction in the course of a total synthesis program, we delineate herein that it has significant strength, both in terms of substrate scope as well as the terminating oxygen nucleophile. As a result, the reaction proved critical in achieving total syntheses of two oxygenated natural products, one of which was prone to over-oxidation. Finally, a mechanistic proposal that accounts for its success is provided.
中文翻译:

用于天然产物合成的基于钯的环化-氧化序列的开发和阐明。
钯催化的序列涉及氧化加成、环化和通过分子间亲核试剂捕获的终止,具有巨大的实用性。事实上,它们可以产生大量具有多种功能的不同多环结构。然而,Pd0 /PdII 变体的一个缺陷是无法将它们与氧基物质结合起来。受到最近在全合成程序过程中发现的一个此类反应的启发,我们在此描述了它在底物范围以及终止氧亲核试剂方面都具有显着的优势。结果,该反应被证明对于实现两种含氧天然产物的全合成至关重要,其中一种容易过度氧化。最后,提供了一个解释其成功的机制建议。
更新日期:2020-01-08
中文翻译:

用于天然产物合成的基于钯的环化-氧化序列的开发和阐明。
钯催化的序列涉及氧化加成、环化和通过分子间亲核试剂捕获的终止,具有巨大的实用性。事实上,它们可以产生大量具有多种功能的不同多环结构。然而,Pd0 /PdII 变体的一个缺陷是无法将它们与氧基物质结合起来。受到最近在全合成程序过程中发现的一个此类反应的启发,我们在此描述了它在底物范围以及终止氧亲核试剂方面都具有显着的优势。结果,该反应被证明对于实现两种含氧天然产物的全合成至关重要,其中一种容易过度氧化。最后,提供了一个解释其成功的机制建议。



























 京公网安备 11010802027423号
京公网安备 11010802027423号