当前位置:
X-MOL 学术
›
Energy Environ. Sci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Revealing electrolyte oxidation via carbonate dehydrogenation on Ni-based oxides in Li-ion batteries by in situ Fourier transform infrared spectroscopy
Energy & Environmental Science ( IF 32.5 ) Pub Date : 2019-11-18 , DOI: 10.1039/c9ee02543j Yirui Zhang 1, 2, 3, 4 , Yu Katayama 2, 3, 4, 5, 6 , Ryoichi Tatara 2, 3, 4, 5 , Livia Giordano 1, 2, 3, 4, 5 , Yang Yu 2, 3, 4, 7 , Dimitrios Fraggedakis 2, 3, 4, 8 , Jame Guangwen Sun 1, 2, 3, 4 , Filippo Maglia 9, 10, 11 , Roland Jung 9, 10, 11 , Martin Z. Bazant 2, 3, 4, 8, 12 , Yang Shao-Horn 1, 2, 3, 4, 5
Energy & Environmental Science ( IF 32.5 ) Pub Date : 2019-11-18 , DOI: 10.1039/c9ee02543j Yirui Zhang 1, 2, 3, 4 , Yu Katayama 2, 3, 4, 5, 6 , Ryoichi Tatara 2, 3, 4, 5 , Livia Giordano 1, 2, 3, 4, 5 , Yang Yu 2, 3, 4, 7 , Dimitrios Fraggedakis 2, 3, 4, 8 , Jame Guangwen Sun 1, 2, 3, 4 , Filippo Maglia 9, 10, 11 , Roland Jung 9, 10, 11 , Martin Z. Bazant 2, 3, 4, 8, 12 , Yang Shao-Horn 1, 2, 3, 4, 5
Affiliation
|
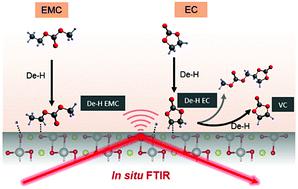
Understanding (electro-)chemical reactions at the electrode–electrolyte interface (EEI) is crucial to promote the cycle life of lithium-ion batteries. In this study, we developed an in situ Fourier-transform infrared spectroscopy (FT-IR) method, which provided unprecedented information on the oxidation of carbonate solvents via dehydrogenation on LiNixMnyCo1−x−yO2 (NMC). While ethylene carbonate (EC) was stable against oxidation on Pt up to 4.8 VLi, unique evidence for dehydrogenation of EC on LiNi0.8Co0.1Mn0.1O2 (NMC811) at voltages as low as 3.8 VLi was revealed by in situ FT-IR measurements, which was supported by density functional theory (DFT) results. Unique dehydrogenated species from EC were observed on NMC811 surface, including dehydrogenated EC anchored on oxides, vinylene carbonate (VC) and dehydrogenated oligomers which could diffuse away from the surface. Similar dehydrogenation on NMC811 was noted for EMC-based and LP57 (1 M LiPF6 in 3 : 7 EC/EMC) electrolytes. In contrast, no dehydrogenation was found for NMC111 or surface-modified NMC by coatings such as Al2O3. In addition, while the dehydrogenation of solvents was observed in 1 M electrolytes with different anions, they were not observed on NMC811 in the concentrated electrolyte (EC/EMC with 3.1 M LiPF6), indicating lithium coordination could suppress dehydrogenation. Dehydrogenation of carbonates on NMC811 accompanied with rapid growth of interfacial impedance with increasing voltage revealed by electrochemical impedance spectroscopy (EIS), while the electrode–electrolyte combinations without dehydrogenation did not show significant impedance growth. Therefore, minimizing carbonate dehydrogenation on the NMC surface by tuning electrode reactivity and electrolyte reactivity is critical to develop high-energy Li-ion batteries with long cycle life.
中文翻译:

原位傅里叶变换红外光谱法研究锂离子电池中镍基氧化物上碳酸盐脱氢引起的电解质氧化
了解电极-电解质界面(EEI)的(电)化学反应对于延长锂离子电池的循环寿命至关重要。在这项研究中,我们开发了一种原位傅立叶变换红外光谱(FT-IR)方法,该方法提供了关于通过LiNi x Mn y Co 1- x - y O 2(NMC)上的脱氢作用来碳酸酯类溶剂氧化的前所未有的信息。碳酸亚乙酯(EC)在高达4.8 V Li的Pt上对氧化稳定,但在LiNi 0.8 Co 0.1 Mn 0.1 O 2上EC脱氢的独特证据(NMC811)在低至3.8 V Li的电压下通过原位FT-IR测量揭示了这一点,这得到密度泛函理论(DFT)结果的支持。在NMC811表面观察到来自EC的独特脱氢物质,包括锚固在氧化物,碳酸亚乙烯酯(VC)和脱氢低聚物上的脱氢EC,这些低聚物可能会从表面扩散出去。对于基于EMC的电解液和LP57(3:7 EC / EMC中的1 M LiPF 6)电解液,在NMC811上也发生了类似的脱氢反应。相反,对于NMC111或表面改性的NMC,未发现通过涂层(如Al 2 O 3)进行脱氢。此外,虽然在具有不同阴离子的1 M电解质中观察到了溶剂的脱氢,但在浓缩电解质(EC / EMC与3.1 M LiPF 6)中的NMC811上未观察到它们的脱氢,表明锂配位可以抑制脱氢。NMC811上碳酸盐的脱氢伴随着界面阻抗的快速增长,而电化学阻抗谱(EIS)显示出电压升高,而没有脱氢的电极-电解质组合并未显示出明显的阻抗增长。因此,通过调节电极反应性和电解质反应性来使NMC表面的碳酸盐脱氢最小化对于开发具有长循环寿命的高能锂离子电池至关重要。
更新日期:2019-11-18
中文翻译:

原位傅里叶变换红外光谱法研究锂离子电池中镍基氧化物上碳酸盐脱氢引起的电解质氧化
了解电极-电解质界面(EEI)的(电)化学反应对于延长锂离子电池的循环寿命至关重要。在这项研究中,我们开发了一种原位傅立叶变换红外光谱(FT-IR)方法,该方法提供了关于通过LiNi x Mn y Co 1- x - y O 2(NMC)上的脱氢作用来碳酸酯类溶剂氧化的前所未有的信息。碳酸亚乙酯(EC)在高达4.8 V Li的Pt上对氧化稳定,但在LiNi 0.8 Co 0.1 Mn 0.1 O 2上EC脱氢的独特证据(NMC811)在低至3.8 V Li的电压下通过原位FT-IR测量揭示了这一点,这得到密度泛函理论(DFT)结果的支持。在NMC811表面观察到来自EC的独特脱氢物质,包括锚固在氧化物,碳酸亚乙烯酯(VC)和脱氢低聚物上的脱氢EC,这些低聚物可能会从表面扩散出去。对于基于EMC的电解液和LP57(3:7 EC / EMC中的1 M LiPF 6)电解液,在NMC811上也发生了类似的脱氢反应。相反,对于NMC111或表面改性的NMC,未发现通过涂层(如Al 2 O 3)进行脱氢。此外,虽然在具有不同阴离子的1 M电解质中观察到了溶剂的脱氢,但在浓缩电解质(EC / EMC与3.1 M LiPF 6)中的NMC811上未观察到它们的脱氢,表明锂配位可以抑制脱氢。NMC811上碳酸盐的脱氢伴随着界面阻抗的快速增长,而电化学阻抗谱(EIS)显示出电压升高,而没有脱氢的电极-电解质组合并未显示出明显的阻抗增长。因此,通过调节电极反应性和电解质反应性来使NMC表面的碳酸盐脱氢最小化对于开发具有长循环寿命的高能锂离子电池至关重要。



























 京公网安备 11010802027423号
京公网安备 11010802027423号