当前位置:
X-MOL 学术
›
Neurotoxicology
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Neuronal specific and non-specific responses to cadmium possibly involved in neurodegeneration: A toxicogenomics study in a human neuronal cell model.
NeuroToxicology ( IF 3.4 ) Pub Date : 2019-11-16 , DOI: 10.1016/j.neuro.2019.11.002 M Forcella 1 , P Lau 2 , M Oldani 1 , P Melchioretto 3 , A Bogni 2 , L Gribaldo 2 , P Fusi 4 , C Urani 3
NeuroToxicology ( IF 3.4 ) Pub Date : 2019-11-16 , DOI: 10.1016/j.neuro.2019.11.002 M Forcella 1 , P Lau 2 , M Oldani 1 , P Melchioretto 3 , A Bogni 2 , L Gribaldo 2 , P Fusi 4 , C Urani 3
Affiliation
|
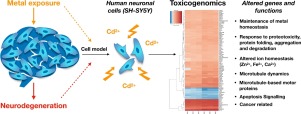
Epidemiological data have linked cadmium exposure to neurotoxicity and to neurodegenerative diseases (e.g., Alzheimer's and Parkinson's disease), and to increased risk of developing ALS. Even though the brain is not a primary target organ, this metal can bypass the blood brain barrier, thus exerting its toxic effects. The coordination chemistry of cadmium is of strong biological relevance, as it resembles to zinc(II) and calcium(II), two ions crucial for neuronal signaling. A toxicogenomics approach applied to a neuronal human model (SH-SY5Y cells) exposed to cadmium (10 and 20 μM) allowed the identification of early deregulated genes and altered processes, and the discrimination between neuronal-specific and unspecific responses as possible triggers of neurodegeneration. Cadmium confirmed its recognized carcinogenicity even on neuronal cells by activating the p53 signaling pathway and genes involved in tumor initiation and cancer cell proliferation, and by down-regulating genes coding for tumor suppressors and for DNA repair enzymes. Two cadmium-induced stress responses were observed: the activation of different members of the heat shock family, as a mechanism to restore protein folding in response to proteotoxicity, and the activation of metallothioneins (MTs), involved in zinc and copper homeostasis, protection against metal toxicity and oxidative damage. Perturbed function of essential metals is suggested by the mineral absorption pathway, with MTs, HMOX1, ZnT-1, and Ferritin genes highly up-regulated. Cadmium interferes also with Ca2+ regulation as S100A2 is one of the top up-regulated genes, coding for a highly specialized family of regulatory Ca2+-binding proteins. Other neuronal-related functions altered in SH-SY5Y cells by cadmium are microtubules dynamics, microtubules motor-based proteins and neuroprotection by down-regulation of NEK3, KIF15, and GREM2 genes, respectively.
中文翻译:

镉可能涉及神经变性的神经元特异性和非特异性反应:在人类神经元细胞模型中的毒理基因组学研究。
流行病学数据已将镉暴露与神经毒性和神经退行性疾病(例如阿尔茨海默氏病和帕金森氏病)联系在一起,并增加了罹患ALS的风险。即使大脑不是主要的目标器官,这种金属也可以绕过血脑屏障,从而发挥其毒性作用。镉的配位化学具有很强的生物学意义,因为它类似于锌(II)和钙(II),这两个离子对于神经元信号传导至关重要。将毒理基因组学方法应用于暴露于镉(10和20μM)的神经元人类模型(SH-SY5Y细胞)可以识别早期失控的基因和改变的过程,并区分神经元特异性和非特异性反应是神经变性的可能诱因。镉通过激活p53信号通路和参与肿瘤起始和癌细胞增殖的基因,并下调编码肿瘤抑制因子和DNA修复酶的基因,从而证实了其对神经元细胞的致癌性。观察到了两种镉诱导的应激反应:热休克家族不同成员的活化,作为恢复蛋白折叠以响应蛋白毒性的一种机制;金属硫蛋白(MTs)的活化,涉及锌和铜的体内平衡,可防止金属毒性和氧化损伤。矿物质吸收途径暗示了必需金属的功能紊乱,其中MT,HMOX1,ZnT-1和铁蛋白基因高度上调。镉也干扰Ca2 +的调节,因为S100A2是最上调的基因之一,编码高度专业的调节性Ca2 +结合蛋白家族。镉在SH-SY5Y细胞中改变的其他与神经元相关的功能分别是微管动力学,微管运动蛋白和通过下调NEK3,KIF15和GREM2基因的神经保护作用。
更新日期:2019-11-16
中文翻译:

镉可能涉及神经变性的神经元特异性和非特异性反应:在人类神经元细胞模型中的毒理基因组学研究。
流行病学数据已将镉暴露与神经毒性和神经退行性疾病(例如阿尔茨海默氏病和帕金森氏病)联系在一起,并增加了罹患ALS的风险。即使大脑不是主要的目标器官,这种金属也可以绕过血脑屏障,从而发挥其毒性作用。镉的配位化学具有很强的生物学意义,因为它类似于锌(II)和钙(II),这两个离子对于神经元信号传导至关重要。将毒理基因组学方法应用于暴露于镉(10和20μM)的神经元人类模型(SH-SY5Y细胞)可以识别早期失控的基因和改变的过程,并区分神经元特异性和非特异性反应是神经变性的可能诱因。镉通过激活p53信号通路和参与肿瘤起始和癌细胞增殖的基因,并下调编码肿瘤抑制因子和DNA修复酶的基因,从而证实了其对神经元细胞的致癌性。观察到了两种镉诱导的应激反应:热休克家族不同成员的活化,作为恢复蛋白折叠以响应蛋白毒性的一种机制;金属硫蛋白(MTs)的活化,涉及锌和铜的体内平衡,可防止金属毒性和氧化损伤。矿物质吸收途径暗示了必需金属的功能紊乱,其中MT,HMOX1,ZnT-1和铁蛋白基因高度上调。镉也干扰Ca2 +的调节,因为S100A2是最上调的基因之一,编码高度专业的调节性Ca2 +结合蛋白家族。镉在SH-SY5Y细胞中改变的其他与神经元相关的功能分别是微管动力学,微管运动蛋白和通过下调NEK3,KIF15和GREM2基因的神经保护作用。



























 京公网安备 11010802027423号
京公网安备 11010802027423号