当前位置:
X-MOL 学术
›
Exp. Neurol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
A novel mouse model for the study of endogenous neural stem and progenitor cells after traumatic brain injury.
Experimental Neurology ( IF 5.3 ) Pub Date : 2019-11-18 , DOI: 10.1016/j.expneurol.2019.113119 Jeremy Anderson 1 , Misaal Patel 1 , Dylan Forenzo 1 , Xin Ai 1 , Catherine Cai 1 , Quinn Wade 1 , Rebecca Risman 1 , Li Cai 1
Experimental Neurology ( IF 5.3 ) Pub Date : 2019-11-18 , DOI: 10.1016/j.expneurol.2019.113119 Jeremy Anderson 1 , Misaal Patel 1 , Dylan Forenzo 1 , Xin Ai 1 , Catherine Cai 1 , Quinn Wade 1 , Rebecca Risman 1 , Li Cai 1
Affiliation
|
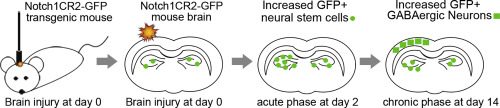
Traumatic brain injury (TBI) is a leading cause of death and disability in the US. Neural stem/progenitor cells (NSPCs) persist in the adult brain and represent a potential cell source for tissue regeneration and wound healing after injury. The Notch signaling pathway is critical for embryonic development and adult brain injury response. However, the specific role of Notch signaling in the injured brain is not well characterized. Our previous study has established a Notch1CR2-GFP reporter mouse line in which the Notch1CR2 enhancer directs GFP expression in NSPCs and their progeny. In this study, we performed closed head injury (CHI) in the Notch1CR2-GFP mice to study the response of injury-activated NSPCs. We show that CHI induces neuroinflammation, cell death, and the expression of typical TBI markers (e.g., ApoE, Il1b, and Tau), validating the animal model. In addition, CHI induces cell proliferation in GFP+ cells expressing NSPC markers, e.g., Notch1 and Nestin. A significant higher percentage of GFP+ astrocytes and GABAergic neurons was observed in the injured brain, with no significant change in oligodendrocyte lineage between the CHI and sham animal groups. Since injury is known to activate astrogliosis, our results suggest that injury-induced GFP+ NSPCs preferentially differentiate into GABAergic neurons. Our study establishes that Notch1CR2-GFP transgenic mouse is a useful tool for the study of NSPC behavior in vivo after TBI. Unveiling the potential of NSPCs response to TBI (e.g., proliferation and differentiation) will identify new therapeutic strategy for the treatment of brain trauma.
中文翻译:

用于研究脑外伤后内源性神经干细胞和祖细胞的新型小鼠模型。
在美国,颅脑外伤(TBI)是导致死亡和残疾的主要原因。神经干/祖细胞(NSPC)持续存在于成年大脑中,代表了损伤后组织再生和伤口愈合的潜在细胞来源。Notch信号通路对于胚胎发育和成人脑损伤反应至关重要。但是,Notch信号在受伤的大脑中的具体作用尚不十分清楚。我们之前的研究建立了一个Notch1CR2-GFP报告基因小鼠系,其中Notch1CR2增强子指导GFP在NSPCs及其子代中的表达。在这项研究中,我们在Notch1CR2-GFP小鼠中进行了闭合性颅脑损伤(CHI),以研究损伤激活的NSPC的反应。我们显示CHI会诱导神经炎症,细胞死亡以及典型TBI标记(例如ApoE,Il1b和Tau)的表达,验证动物模型。此外,CHI可诱导表达NSPC标记(例如Notch1和Nestin)的GFP +细胞中的细胞增殖。在受伤的大脑中观察到明显更高的GFP +星形胶质细胞和GABA能神经元百分比,在CHI和假动物组之间的少突胶质细胞谱系没有显着变化。由于已知损伤会激活星形胶质细胞增生,因此我们的结果表明,损伤诱导的GFP + NSPCs优先分化为GABA能神经元。我们的研究表明,Notch1CR2-GFP转基因小鼠是研究TBI后体内NSPC行为的有用工具。揭示NSPC对TBI(例如,增殖和分化)反应的潜力将为脑创伤的治疗确定新的治疗策略。CHI在表达NSPC标记(例如Notch1和Nestin)的GFP +细胞中诱导细胞增殖。在受伤的大脑中观察到明显更高的GFP +星形胶质细胞和GABA能神经元百分比,在CHI和假动物组之间的少突胶质细胞谱系没有显着变化。由于已知损伤会激活星形胶质细胞增生,因此我们的结果表明,损伤诱导的GFP + NSPCs优先分化为GABA能神经元。我们的研究表明,Notch1CR2-GFP转基因小鼠是研究TBI后体内NSPC行为的有用工具。揭示NSPC对TBI(例如,增殖和分化)反应的潜力将为脑创伤的治疗确定新的治疗策略。CHI在表达NSPC标记(例如Notch1和Nestin)的GFP +细胞中诱导细胞增殖。在受伤的大脑中观察到明显更高的GFP +星形胶质细胞和GABA能神经元百分比,在CHI和假动物组之间的少突胶质细胞谱系没有显着变化。由于已知损伤会激活星形胶质细胞增生,因此我们的结果表明,损伤诱导的GFP + NSPCs优先分化为GABA能神经元。我们的研究表明,Notch1CR2-GFP转基因小鼠是研究TBI后体内NSPC行为的有用工具。揭示NSPC对TBI(例如,增殖和分化)反应的潜力将为脑创伤的治疗确定新的治疗策略。在受伤的大脑中观察到明显更高的GFP +星形胶质细胞和GABA能神经元百分比,CHI和假动物组之间的少突胶质细胞谱系没有显着变化。由于已知损伤会激活星形胶质细胞增生,因此我们的结果表明,损伤诱导的GFP + NSPCs优先分化为GABA能神经元。我们的研究表明,Notch1CR2-GFP转基因小鼠是研究TBI后体内NSPC行为的有用工具。揭示NSPC对TBI(例如,增殖和分化)反应的潜力将确定治疗脑外伤的新治疗策略。在受伤的大脑中观察到明显更高的GFP +星形胶质细胞和GABA能神经元百分比,在CHI和假动物组之间的少突胶质细胞谱系没有显着变化。由于已知损伤会激活星形胶质细胞增生,因此我们的结果表明,损伤诱导的GFP + NSPCs优先分化为GABA能神经元。我们的研究表明,Notch1CR2-GFP转基因小鼠是研究TBI后体内NSPC行为的有用工具。揭示NSPC对TBI(例如,增殖和分化)反应的潜力将为脑创伤的治疗确定新的治疗策略。我们的结果表明,损伤诱导的GFP + NSPCs优先分化为GABA能神经元。我们的研究表明,Notch1CR2-GFP转基因小鼠是研究TBI后体内NSPC行为的有用工具。揭示NSPC对TBI(例如,增殖和分化)反应的潜力将为脑创伤的治疗确定新的治疗策略。我们的结果表明,损伤诱导的GFP + NSPCs优先分化为GABA能神经元。我们的研究表明,Notch1CR2-GFP转基因小鼠是研究TBI后体内NSPC行为的有用工具。揭示NSPC对TBI(例如,增殖和分化)反应的潜力将为脑创伤的治疗确定新的治疗策略。
更新日期:2019-11-18
中文翻译:

用于研究脑外伤后内源性神经干细胞和祖细胞的新型小鼠模型。
在美国,颅脑外伤(TBI)是导致死亡和残疾的主要原因。神经干/祖细胞(NSPC)持续存在于成年大脑中,代表了损伤后组织再生和伤口愈合的潜在细胞来源。Notch信号通路对于胚胎发育和成人脑损伤反应至关重要。但是,Notch信号在受伤的大脑中的具体作用尚不十分清楚。我们之前的研究建立了一个Notch1CR2-GFP报告基因小鼠系,其中Notch1CR2增强子指导GFP在NSPCs及其子代中的表达。在这项研究中,我们在Notch1CR2-GFP小鼠中进行了闭合性颅脑损伤(CHI),以研究损伤激活的NSPC的反应。我们显示CHI会诱导神经炎症,细胞死亡以及典型TBI标记(例如ApoE,Il1b和Tau)的表达,验证动物模型。此外,CHI可诱导表达NSPC标记(例如Notch1和Nestin)的GFP +细胞中的细胞增殖。在受伤的大脑中观察到明显更高的GFP +星形胶质细胞和GABA能神经元百分比,在CHI和假动物组之间的少突胶质细胞谱系没有显着变化。由于已知损伤会激活星形胶质细胞增生,因此我们的结果表明,损伤诱导的GFP + NSPCs优先分化为GABA能神经元。我们的研究表明,Notch1CR2-GFP转基因小鼠是研究TBI后体内NSPC行为的有用工具。揭示NSPC对TBI(例如,增殖和分化)反应的潜力将为脑创伤的治疗确定新的治疗策略。CHI在表达NSPC标记(例如Notch1和Nestin)的GFP +细胞中诱导细胞增殖。在受伤的大脑中观察到明显更高的GFP +星形胶质细胞和GABA能神经元百分比,在CHI和假动物组之间的少突胶质细胞谱系没有显着变化。由于已知损伤会激活星形胶质细胞增生,因此我们的结果表明,损伤诱导的GFP + NSPCs优先分化为GABA能神经元。我们的研究表明,Notch1CR2-GFP转基因小鼠是研究TBI后体内NSPC行为的有用工具。揭示NSPC对TBI(例如,增殖和分化)反应的潜力将为脑创伤的治疗确定新的治疗策略。CHI在表达NSPC标记(例如Notch1和Nestin)的GFP +细胞中诱导细胞增殖。在受伤的大脑中观察到明显更高的GFP +星形胶质细胞和GABA能神经元百分比,在CHI和假动物组之间的少突胶质细胞谱系没有显着变化。由于已知损伤会激活星形胶质细胞增生,因此我们的结果表明,损伤诱导的GFP + NSPCs优先分化为GABA能神经元。我们的研究表明,Notch1CR2-GFP转基因小鼠是研究TBI后体内NSPC行为的有用工具。揭示NSPC对TBI(例如,增殖和分化)反应的潜力将为脑创伤的治疗确定新的治疗策略。在受伤的大脑中观察到明显更高的GFP +星形胶质细胞和GABA能神经元百分比,CHI和假动物组之间的少突胶质细胞谱系没有显着变化。由于已知损伤会激活星形胶质细胞增生,因此我们的结果表明,损伤诱导的GFP + NSPCs优先分化为GABA能神经元。我们的研究表明,Notch1CR2-GFP转基因小鼠是研究TBI后体内NSPC行为的有用工具。揭示NSPC对TBI(例如,增殖和分化)反应的潜力将确定治疗脑外伤的新治疗策略。在受伤的大脑中观察到明显更高的GFP +星形胶质细胞和GABA能神经元百分比,在CHI和假动物组之间的少突胶质细胞谱系没有显着变化。由于已知损伤会激活星形胶质细胞增生,因此我们的结果表明,损伤诱导的GFP + NSPCs优先分化为GABA能神经元。我们的研究表明,Notch1CR2-GFP转基因小鼠是研究TBI后体内NSPC行为的有用工具。揭示NSPC对TBI(例如,增殖和分化)反应的潜力将为脑创伤的治疗确定新的治疗策略。我们的结果表明,损伤诱导的GFP + NSPCs优先分化为GABA能神经元。我们的研究表明,Notch1CR2-GFP转基因小鼠是研究TBI后体内NSPC行为的有用工具。揭示NSPC对TBI(例如,增殖和分化)反应的潜力将为脑创伤的治疗确定新的治疗策略。我们的结果表明,损伤诱导的GFP + NSPCs优先分化为GABA能神经元。我们的研究表明,Notch1CR2-GFP转基因小鼠是研究TBI后体内NSPC行为的有用工具。揭示NSPC对TBI(例如,增殖和分化)反应的潜力将为脑创伤的治疗确定新的治疗策略。


























 京公网安备 11010802027423号
京公网安备 11010802027423号