Signal Transduction and Targeted Therapy ( IF 39.3 ) Pub Date : 2019-11-18 , DOI: 10.1038/s41392-019-0084-3 YongHao Li 1 , Xiong Liu 2 , Xian Lin 3 , Menyang Zhao 1 , Yanyi Xiao 1 , Chen Liu 1 , Zixi Liang 1 , Zelong Lin 1 , Renhui Yi 1 , Zibo Tang 1 , Jiahao Liu 1 , Xin Li 3 , Qingping Jiang 4 , Libo Li 1 , Yinyin Xie 1 , Zhen Liu 1, 5 , Weiyi Fang 1
|
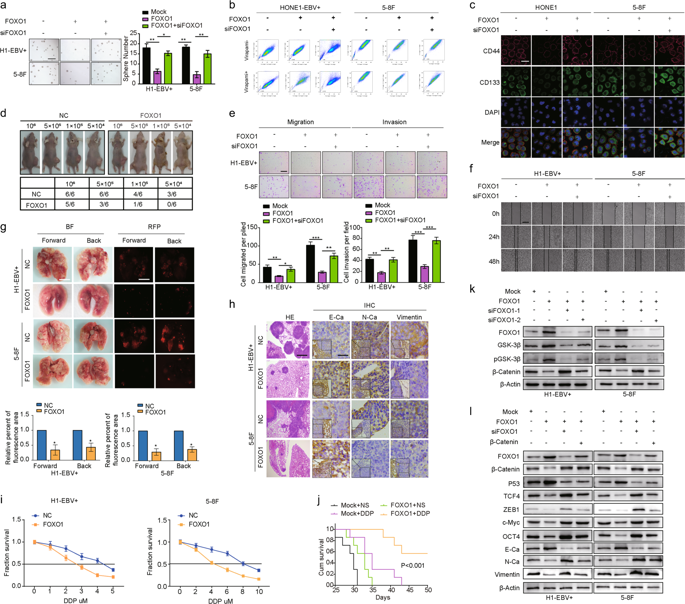
In this study, we present novel molecular mechanisms by which FOXO1 functions as a tumor suppressor to prevent the pathogenesis of nasopharyngeal carcinoma (NPC). First, we observed that FOXO1 not only controlled tumor stemness and metastasis, but also sensitized NPC cells to cisplatin (DDP) in vitro and in vivo. Mechanistic studies demonstrated that FOXO1-induced miR-200b expression through the GSK3β/β-catenin/TCF4 network-mediated stimulation of ZEB1, which reduced tumor stemness and the epithelial–mesenchymal transition (EMT) signal. Furthermore, we observed FOXO1 interaction with MYH9 and suppression of MYH9 expression by modulating the PI3K/AKT/c-Myc/P53/miR-133a-3p pathway. Decreased MYH9 expression not only reduced its interactions with GSK3β, but also attenuated TRAF6 expression, which then decreased the ubiquitin-mediated degradation of GSK3β protein. Increased GSK3β expression stimulated the β-catenin/TCF4/ZEB1/miR-200b network, which increased the downstream tumor stemness and EMT signals. Subsequently, we observed that chemically synthesized cinobufotalin (CB) strongly increased FOXO1-induced DDP chemosensitivity by reducing MYH9 expression, and the reduction in MYH9 modulated GSK3β/β-catenin and its downstream tumor stemness and EMT signal in NPC. In clinical samples, the combination of low FOXO1 expression and high MYH9 expression indicated the worst overall survival rates. Our studies demonstrated that CB potently induced FOXO1-mediated DDP sensitivity by antagonizing its binding partner MYH9 to modulate tumor stemness in NPC.
中文翻译:

化合物华蟾素通过拮抗其结合伴侣 MYH9 有效诱导 FOXO1 刺激的顺铂敏感性
在这项研究中,我们提出了 FOXO1 作为肿瘤抑制因子预防鼻咽癌 (NPC) 发病机制的新分子机制。首先,我们观察到FOXO1不仅控制肿瘤干性和转移,而且在体外和体内使NPC细胞对顺铂(DDP)敏感。机制研究表明,FOXO1 通过 GSK3β/β-catenin/TCF4 网络介导的 ZEB1 刺激诱导 miR-200b 表达,从而降低肿瘤干性和上皮间质转化 (EMT) 信号。此外,我们观察到 FOXO1 与 MYH9 相互作用,并通过调节 PI3K/AKT/c-Myc/P53/miR-133a-3p 通路抑制 MYH9 表达。MYH9 表达的减少不仅减少了其与 GSK3β 的相互作用,而且还减弱了 TRAF6 的表达,从而减少了泛素介导的 GSK3β 蛋白的降解。GSK3β表达增加刺激了β-连环蛋白/TCF4/ZEB1/miR-200b网络,从而增加了下游肿瘤干性和EMT信号。随后,我们观察到化学合成的华蟾素(CB)通过减少 MYH9 的表达来强烈增加 FOXO1 诱导的 DDP 化疗敏感性,并且 MYH9 的减少调节 GSK3β/β-catenin 及其下游肿瘤干性和 NPC 中的 EMT 信号。在临床样本中,低 FOXO1 表达和高 MYH9 表达的组合表明总生存率最差。我们的研究表明,CB 通过拮抗 FOXO1 介导的 DDP 敏感性,通过拮抗其结合伴侣 MYH9 来调节 NPC 的肿瘤干性。



























 京公网安备 11010802027423号
京公网安备 11010802027423号