Catalysis Communications ( IF 3.7 ) Pub Date : 2019-11-18 , DOI: 10.1016/j.catcom.2019.105891 Daniel Mack , Lia-Sabrina Berthold , Yvonne Traa , Elias Klemm
|
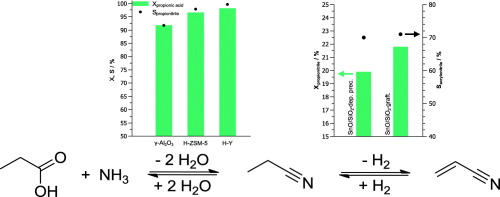
Acrylonitrile is currently made from fossil resources. We investigated the synthesis of acrylonitrile from propionic acid in only two steps, which opens up a new route to acrylonitrile based on propionic acid obtained by fermentation. Propionic acid was initially converted to propionitrile with NH3 at 400 °C. Zeolite HY allows for >99% selectivity at 98% conversion. However, coking led to deactivation after 180 min on stream. The dehydrogenation of propionitrile to acrylonitrile was catalyzed by SnO/SiO2. The selectivity was 71% at 22% conversion, which gives already after first experiments an overall selectivity for acrylonitrile production from propionic acid of 71%.
中文翻译:

由丙酸生产丙烯腈的新两步途径
丙烯腈目前由化石资源制成。我们仅用两个步骤就研究了由丙酸合成丙烯腈的方法,这为通过发酵获得的丙酸开辟了一条通往丙烯腈的新途径。丙酸最初在400°C下用NH 3转化为丙腈。沸石H Y在98%的转化率下可实现> 99%的选择性。但是,焦化导致在运行180分钟后失活。SnO / SiO 2催化丙腈脱氢制丙烯腈。在22%的转化率下,选择性为71%,这在首次实验后已经使由丙酸生产丙烯腈的总选择性为71%。


























 京公网安备 11010802027423号
京公网安备 11010802027423号