当前位置:
X-MOL 学术
›
J. Chem. Thermodyn.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Equilibrium solubility determination, solvent effect and preferential solvation of amoxicillin in aqueous co-solvent mixtures of N,N-dimethylformamide, isopropanol, N-methyl pyrrolidone and ethylene glycol
The Journal of Chemical Thermodynamics ( IF 2.6 ) Pub Date : 2020-01-01 , DOI: 10.1016/j.jct.2019.106010 Wanxin Li , Ali Farajtabar , Rong Xing , Yiting Zhu , Hongkun Zhao
The Journal of Chemical Thermodynamics ( IF 2.6 ) Pub Date : 2020-01-01 , DOI: 10.1016/j.jct.2019.106010 Wanxin Li , Ali Farajtabar , Rong Xing , Yiting Zhu , Hongkun Zhao
|
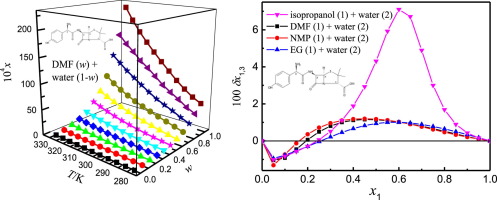
Abstract The mole fraction solubility of amoxicillin in four co-solvent mixtures of N,N-dimethylformamide (DMF) + water (2), isopropanol (1) + water (2), N-methyl pyrrolidone (NMP) (1) + water (2) and ethylene glycol (EG, 1) + water (2) at temperatures from 278.15 K to 328.15 K was determined by means of the shake-flask technique. At the same temperature and composition of DMF, isopropanol, NMP or EG, the solubility magnitude of amoxicillin was highest in the DMF (1) + water (2) mixture, and lowest in the isopropanol (1) + water (2) mixture. Through the Jouyban-Acree model, amoxicillin solubility was well correlated obtaining RAD lower than 4.55% and RMSD lower than 1.96 × 10-4. Quantitative values for the local mole fraction of DMF (isopropanol, NMP or EG) and water nearby the amoxicillin were computed by means of the Inverse Kirkwood–Buff integrals method. In the DMF (1) + water (2) mixture with compositions 0.20
中文翻译:

阿莫西林在 N,N-二甲基甲酰胺、异丙醇、N-甲基吡咯烷酮和乙二醇的水性共溶剂混合物中的平衡溶解度测定、溶剂效应和优先溶剂化
摘要 阿莫西林在 N,N-二甲基甲酰胺 (DMF) + 水 (2)、异丙醇 (1) + 水 (2)、N-甲基吡咯烷酮 (NMP) (1) + 水 4 种共溶剂混合物中的摩尔分数溶解度(2) 和乙二醇 (EG, 1) + 水 (2) 在 278.15 K 至 328.15 K 的温度下通过摇瓶技术测定。在DMF、异丙醇、NMP或EG的相同温度和组成下,阿莫西林在DMF(1)+水(2)混合物中的溶解度大小最高,在异丙醇(1)+水(2)混合物中最低。通过 Jouyban-Acree 模型,阿莫西林溶解度相关性良好,获得的 RAD 低于 4.55%,RMSD 低于 1.96 × 10-4。DMF(异丙醇,NMP 或 EG) 和阿莫西林附近的水是通过逆柯克伍德-布夫积分方法计算的。在 DMF (1) + 水 (2) 混合物中,组成为 0.20
更新日期:2020-01-01
中文翻译:

阿莫西林在 N,N-二甲基甲酰胺、异丙醇、N-甲基吡咯烷酮和乙二醇的水性共溶剂混合物中的平衡溶解度测定、溶剂效应和优先溶剂化
摘要 阿莫西林在 N,N-二甲基甲酰胺 (DMF) + 水 (2)、异丙醇 (1) + 水 (2)、N-甲基吡咯烷酮 (NMP) (1) + 水 4 种共溶剂混合物中的摩尔分数溶解度(2) 和乙二醇 (EG, 1) + 水 (2) 在 278.15 K 至 328.15 K 的温度下通过摇瓶技术测定。在DMF、异丙醇、NMP或EG的相同温度和组成下,阿莫西林在DMF(1)+水(2)混合物中的溶解度大小最高,在异丙醇(1)+水(2)混合物中最低。通过 Jouyban-Acree 模型,阿莫西林溶解度相关性良好,获得的 RAD 低于 4.55%,RMSD 低于 1.96 × 10-4。DMF(异丙醇,NMP 或 EG) 和阿莫西林附近的水是通过逆柯克伍德-布夫积分方法计算的。在 DMF (1) + 水 (2) 混合物中,组成为 0.20


























 京公网安备 11010802027423号
京公网安备 11010802027423号