当前位置:
X-MOL 学术
›
BBA Biomembr.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Influence of interfacial tryptophan residues on an arginine-flanked transmembrane helix.
Biochimica et Biophysica Acta (BBA) - Biomembranes ( IF 3.4 ) Pub Date : 2019-11-16 , DOI: 10.1016/j.bbamem.2019.183134 Sara J Sustich 1 , Fahmida Afrose 1 , Denise V Greathouse 1 , Roger E Koeppe 1
Biochimica et Biophysica Acta (BBA) - Biomembranes ( IF 3.4 ) Pub Date : 2019-11-16 , DOI: 10.1016/j.bbamem.2019.183134 Sara J Sustich 1 , Fahmida Afrose 1 , Denise V Greathouse 1 , Roger E Koeppe 1
Affiliation
|
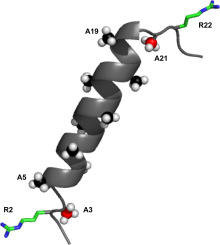
The transmembrane helices of membrane proteins often are flanked by interfacial charged or aromatic residues that potentially help to anchor the membrane-spanning protein. For isolated single-span helices, the interfacial residues may be especially important for stabilizing particular tilted transmembrane orientations. The peptide RWALP23 (acetyl-GR2ALW(LA)6LWLAR22A-amide) has been employed to investigate the interplay between interfacial arginines and tryptophans. Here we replace the tryptophans of RWALP23 with A5 and A19, to investigate arginines alone with respect to helix fraying and orientation in varying lipid bilayers. Deuterated alanines incorporated into the central sequence allow the orientation and stability of the core helix to be assessed by means of solid -state 2H NMR in bilayers of DOPC, DMPC and DLPC. The helix tilt from the bilayer normal is found to increase slightly when R2 and R22 are present, and increases still further when the tryptophans W5 and W19 are replaced by alanines. The extent of helix dynamic averaging remains low in all cases. The preferred helix azimuthal rotation is essentially constant for all of the helices in each of the lipid membranes considered here. The alanines located outside of the core region of the peptide are sensitive to helical integrity. The new alanines, A5 and A19, therefore, provide new information about the length of the core helix and the onset of unraveling of the terminals. Residue A19 remains essentially on the central helix in each lipid membrane, while residues A3, A5 and A21 deviate from the core helix to an extent that depends on the membrane thickness. Differential unraveling of the two ends to expose peptide backbone groups for hydrogen bonding therefore acts together with specific interfacial side chains to stabilize a transmembrane helix.
中文翻译:

界面色氨酸残基对精氨酸侧翼跨膜螺旋的影响。
膜蛋白的跨膜螺旋通常位于界面带电或芳族残基的两侧,这可能有助于锚定跨膜蛋白。对于孤立的单跨螺旋,界面残基对于稳定特定的倾斜跨膜取向可能尤其重要。肽RWALP23(乙酰基-GR2ALW(LA)6LWLAR22A-酰胺)已被用于研究界面精氨酸和色氨酸之间的相互作用。在这里,我们用A5和A19代替RWALP23的色氨酸,以研究精氨酸在不同脂质双层中对螺旋磨损和取向的影响。并入中心序列的氘代丙氨酸使核心螺旋的取向和稳定性可以通过在DOPC,DMPC和DLPC双层中的固态2 H NMR进行评估。当存在R2和R22时,发现从双层法线的螺旋倾斜稍微增加,并且当色氨酸W5和W19被丙氨酸替代时,螺旋倾斜的倾斜进一步增加。在所有情况下,螺旋动态平均的程度都较低。对于这里考虑的每个脂质膜中的所有螺旋,优选的螺旋方位角旋转基本上是恒定的。位于肽核心区域外部的丙氨酸对螺旋完整性敏感。因此,新的丙氨酸A5和A19提供了有关核心螺旋长度和端子解开的新信息。残基A19基本上保留在每个脂质膜的中央螺旋上,而残基A3,A5和A21偏离核心螺旋的程度取决于膜的厚度。
更新日期:2019-11-16
中文翻译:

界面色氨酸残基对精氨酸侧翼跨膜螺旋的影响。
膜蛋白的跨膜螺旋通常位于界面带电或芳族残基的两侧,这可能有助于锚定跨膜蛋白。对于孤立的单跨螺旋,界面残基对于稳定特定的倾斜跨膜取向可能尤其重要。肽RWALP23(乙酰基-GR2ALW(LA)6LWLAR22A-酰胺)已被用于研究界面精氨酸和色氨酸之间的相互作用。在这里,我们用A5和A19代替RWALP23的色氨酸,以研究精氨酸在不同脂质双层中对螺旋磨损和取向的影响。并入中心序列的氘代丙氨酸使核心螺旋的取向和稳定性可以通过在DOPC,DMPC和DLPC双层中的固态2 H NMR进行评估。当存在R2和R22时,发现从双层法线的螺旋倾斜稍微增加,并且当色氨酸W5和W19被丙氨酸替代时,螺旋倾斜的倾斜进一步增加。在所有情况下,螺旋动态平均的程度都较低。对于这里考虑的每个脂质膜中的所有螺旋,优选的螺旋方位角旋转基本上是恒定的。位于肽核心区域外部的丙氨酸对螺旋完整性敏感。因此,新的丙氨酸A5和A19提供了有关核心螺旋长度和端子解开的新信息。残基A19基本上保留在每个脂质膜的中央螺旋上,而残基A3,A5和A21偏离核心螺旋的程度取决于膜的厚度。



























 京公网安备 11010802027423号
京公网安备 11010802027423号