The Lancet HIV ( IF 16.1 ) Pub Date : 2019-11-15 , DOI: 10.1016/s2352-3018(19)30340-6 Paige L Williams 1 , Cenk Yildirim 1 , Ellen G Chadwick 2 , Russell B Van Dyke 3 , Renee Smith 4 , Katharine F Correia 5 , Alexandria DiPerna 6 , George R Seage 1 , Rohan Hazra 7 , Claudia S Crowell 8 ,
|
Background
Perinatal HIV transmission has substantially decreased with combination antiretroviral regimens, but complications in children who are HIV-exposed but uninfected, such as microcephaly, warrant ongoing surveillance. We aimed to evaluate whether individual in utero antiretroviral exposures were associated with increased risk of microcephaly based on long-term follow-up of infants and children who are HIV-exposed but uninfected.
Methods
We evaluated children aged younger than 18 years who were HIV-exposed but uninfected with at least one head circumference measurement while enrolled in the Surveillance Monitoring for ART Toxicities (SMARTT) study at 22 clinical sites in the USA, including Puerto Rico. This prospective cohort study was done by the Pediatric HIV/AIDS Cohort Study network. Microcephaly was defined as having a head circumference Z score <–2 according to the 2000 US Centers for Disease Control and Prevention growth charts for children 6–36 months old and according to Nellhaus standards (head circumference <2nd percentile) after 36 months (SMARTT criteria); an alternate definition for microcephaly was based on applying Nellhaus standards across all ages (Nellhaus criteria). Modified Poisson regression models were fit to obtain relative risks (RRs) for associations between in utero antiretroviral exposure and microcephaly status, adjusted for potential confounders. Neurodevelopmental functioning was compared in children who are HIV-exposed but uninfected with or without microcephaly.
Findings
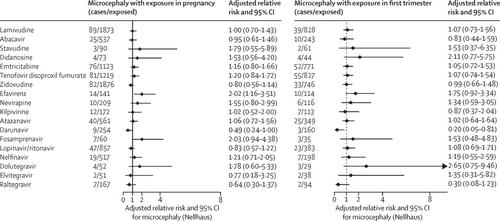
Between March 21, 2007, and Aug 1, 2017, 3055 participants enrolled in SMARTT had at least one head circumference measurement. The cumulative incidence of microcephaly over a median of 5·1 years of follow-up (IQR 3·0–7·2) was 159 (5·2%, 95% CI 4·4–6·1) by Nellhaus criteria and 70 (2·3%, 1·8–2·9) by SMARTT criteria. In adjusted models, in utero exposure to efavirenz (4·7% exposed) was associated with increased risk of microcephaly by both Nellhaus standards (adjusted RR 2·02, 95% CI 1·16–3·51) and SMARTT criteria (2·56, 1·22–5·37). These associations were more pronounced in children exposed to combination regimens of efavirenz that included zidovudine plus lamivudine than those including tenofovir plus emtricitabine. Protective associations were observed for darunavir exposure (adjusted RR 0·50, 95% CI 0·24–1·00). Children who are HIV-exposed but uninfected with microcephaly had lower mean scores on neurodevelopmental assessments at age 1 and 5 years and a higher prevalence of neurodevelopmental impairment than those without microcephaly.
Interpretation
These findings support consideration of alternatives to efavirenz as part of first-line antiretroviral therapy for pregnant women.
Funding
Eunice Kennedy Shriver National Institute of Child Health and Human Development.
中文翻译:

艾滋病毒暴露但未感染儿童的母亲抗逆转录病毒使用与小头畸形的关系:一项前瞻性队列研究。
背景
联合使用抗逆转录病毒疗法可明显降低围产期艾滋病毒的传播,但是,暴露于艾滋病毒但未感染的儿童的并发症(如小头畸形)需要进行持续监测。我们旨在通过对长期暴露于HIV感染但未感染的婴儿和儿童进行随访,评估子宫内抗逆转录病毒暴露的个体是否与小头畸形风险增加相关。
方法
我们纳入了包括波多黎各在内的美国22个临床场所的ART毒性监测监测(SMARTT)研究,评估了18岁以下未接受HIV感染但未感染头围的儿童。这项前瞻性队列研究是由儿童HIV / AIDS队列研究网络完成的。根据2000年美国疾病预防控制中心成长图,对于6至36个月大的儿童,以及根据Nellhaus标准(36岁后的头围<2%),小头畸形被定义为头围Z得分<–2。标准); 小头畸形的另一种定义是基于在所有年龄段都适用Nellhaus标准(Nellhaus标准)。修改后的Poisson回归模型适合获得子宫内抗逆转录病毒暴露与小头畸形之间相关性的相对风险(RR),并针对潜在的混杂因素进行了调整。比较了暴露于艾滋病毒但未感染或患有小头畸形的儿童的神经发育功能。
发现
在2007年3月21日至2017年8月1日之间,参加SMARTT的3055名参与者至少进行了一次头围测量。根据Nellhaus标准,在平均5·1年的随访(IQR 3·0-7·2)中,小头畸形的累积发生率为159(5·2%,95%CI 4·4-6·1)。根据SMARTT标准,为70(2·3%,1·8–2·9)。在调整后的模型中,Nellhaus标准(调整后的RR 2·02、95%CI 1·16–3·51)和SMARTT标准(2)使子宫内暴露于依非韦伦(4.7%暴露)与小头畸形风险增加相关。 ·56,1·22–5·37)。这些接触在接受依非韦伦联合西多夫定加拉米夫定联合治疗的儿童中比包括替诺福韦加恩曲他滨的儿童更明显。观察到达那那韦暴露具有保护性关联(调整后的RR 0·50、95%CI 0·24-1·00)。
解释
这些发现支持考虑将依非韦伦替代品作为孕妇一线抗逆转录病毒疗法的一部分。
资金
Eunice Kennedy Shriver国家儿童健康与人类发展研究所。


























 京公网安备 11010802027423号
京公网安备 11010802027423号