The Lancet HIV ( IF 16.1 ) Pub Date : 2019-11-15 , DOI: 10.1016/s2352-3018(19)30336-4 Jean-Michel Molina 1 , Kathleen Squires 2 , Paul E Sax 3 , Pedro Cahn 4 , Johan Lombaard 5 , Edwin DeJesus 6 , Ming-Tain Lai 7 , Anthony Rodgers 7 , Lisa Lupinacci 7 , Sushma Kumar 7 , Peter Sklar 7 , George J Hanna 7 , Carey Hwang 7 , Elizabeth Anne Martin 7 ,
|
Background
Doravirine is a novel, non-nucleoside reverse transcriptase inhibitor that has shown non-inferior efficacy to ritonavir-boosted darunavir, with a superior lipid profile, in adults with HIV who were treatment naive at week 48 in the phase 3 DRIVE-FORWARD trial. Here we present the 96-week data for the study.
Methods
This randomised, controlled, double-blind, multicentre, non-inferiority, phase 3 study was undertaken at 125 clinical centres in 15 countries. Eligible participants were adults (aged ≥18 years) infected with HIV-1 who were naive to antiretroviral therapy, with a plasma HIV-1 RNA concentration of 1000 copies per mL or higher at screening, and no known resistance to any of the study drugs. Participants were randomly assigned (1:1) using an interactive voice and web response system, stratified by baseline HIV-1 RNA concentration and background nucleoside reverse transcriptase inhibitor therapy, to doravirine (100 mg per day) or ritonavir-boosted darunavir (100 mg ritonavir and 800 mg darunavir per day), both with investigator-selected nucleoside reverse transcriptase inhibitors: emtricitabine and tenofovir disoproxil fumarate or abacavir and lamivudine. Participants and investigators were masked to treatment assignment until week 96. The primary efficacy endpoint was the proportion of participants who had a plasma HIV-1 RNA concentration of less than 50 copies per mL at week 48, which has been reported previously. Here we report the key secondary efficacy endpoint of the proportion of participants who achieved this concentration by week 96, assessed in all participants who received at least one dose of any study drug, regardless of whether it was their randomly assigned treatment. We used a US Food and Drug Administration snapshot approach and a margin of 10 percentage points to define the non-inferiority of doravirine to ritonavir-boosted darunavir at 96 weeks. Key safety endpoints were change in fasting serum lipid concentrations, the incidence of adverse events, and time to discontinuation due to an adverse event, assessed in all participants who received at least one dose of any study medication. This study is registered with ClinicalTrials.gov, NCT02275780, and is closed to accrual.
Findings
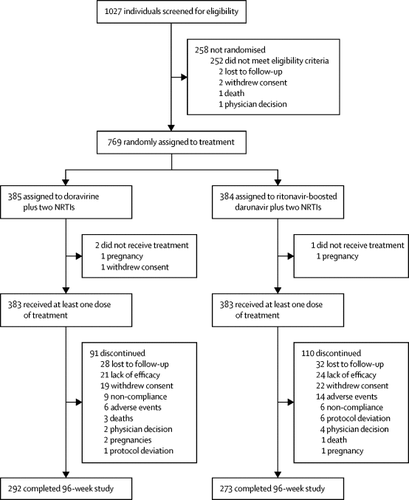
Between Dec 1, 2014, and Oct 20, 2015, 1027 individuals were screened, of whom 769 participants were randomly assigned to doravirine (n=385) or ritonavir-boosted darunavir (n=384), and 383 in both groups were given at least one dose of their allocated treatment. Most participants were male (645 [84%] of 766) and white (560 [73%]), with a mean age of 35·2 years (SD 10·6). 292 participants in the doravirine group and 273 in the darunavir group completed 96 weeks of treatment. At week 96, a higher proportion of the doravirine group (277 [73%] of 383) achieved an HIV-1 RNA concentration of less than 50 copies per mL than did of the darunavir group (248 [66%] of 383; difference 7·1%, 95% CI 0·5–13·7). Responses were similar regardless of baseline characteristics. Treatment-emergent resistance to any study drug occurred in two (1%) of 383 participants in the doravirine group and one (<1%) of 383 in the ritonavir-boosted darunavir group. Significant differences were seen between treatment groups in mean changes from baseline in LDL cholesterol (−14·6 mg/dL, 95% CI −18·2 to −11·0) and non-HDL cholesterol (−18·4 mg/dL, −22·5 to −14·3). Frequencies of adverse events were similar between groups. No significant treatment difference (log-rank nominal p=0·063) through week 96 was observed in time to discontinuation due to an adverse event. The most common adverse events (week 0–96) were diarrhoea (65 [17%] in the doravirine group vs 91 [24%] in the ritonavir-boosted darunavir group), nausea (45 [12%] vs 52 [14%]), headache (57 [15%] vs 46 [12%]), and upper respiratory tract infection (51 [13%] vs 30 [8%]). Two participants, one in each group, died during treatment; neither death was considered to be related to study medication.
Interpretation
These results through 96 weeks support the efficacy and safety results reported previously for doravirine at 48 weeks, supporting the use of doravirine for the long-term treatment of adults with previously untreated HIV-1 infection.
Funding
Merck.
中文翻译:

天真抗艾滋病毒成人HIV-1的多拉维林与利托那韦强化的达那那韦的比较(DRIVE-FORWARD):一项随机,双盲,非劣效,3期临床试验的96周结果。
背景
Doravirine是一种新型的非核苷类逆转录酶抑制剂,在3期DRIVE-FORWARD试验的第48周未接受过治疗的成人HIV感染者中,对利托那韦增强的darunavir的血脂水平没有较差的疗效,并且脂质水平更高。在这里,我们介绍了这项研究的96周数据。
方法
这项随机,对照,双盲,多中心,非自卑性的3期研究在15个国家/地区的125个临床中心进行。合格的参与者是未接受抗逆转录病毒治疗的HIV-1感染的成年人(≥18岁),筛查时血浆HIV-1 RNA浓度为1000拷贝/ mL或更高,并且对任何一种研究药物均无已知耐药性。使用交互式语音和网络响应系统将参与者随机分配(1:1),按基线HIV-1 RNA浓度和背景核苷逆转录酶抑制剂治疗分层,将其分配给doravirine(每天100 mg)或ritonavir增强的darunavir(100 mg)利托那韦和每天800毫克地那韦),均与研究者选择的核苷类逆转录酶抑制剂同时使用:恩曲他滨和替诺福韦富马酸酯或阿巴卡韦和拉米夫定。参与者和研究者在第96周之前均无法接受治疗。主要疗效终点是在第48周时血浆HIV-1 RNA浓度低于50拷贝/ mL的参与者比例。在此,我们报告了在第96周达到此浓度的参与者比例的关键次要疗效终点,该比例在接受至少一剂任何研究药物的所有参与者中进行了评估,无论其是否是随机分配的治疗。我们使用了美国食品药品监督管理局的快照方法,并以10个百分点的差值定义了在96周时,doravirine对ritonavir增强的darunavir的非劣效性。在所有接受至少一种剂量的任何研究药物的受试者中,评估的主要安全性终点为空腹血清脂质浓度变化,不良事件的发生率以及因不良事件导致停药的时间。该研究已在ClinicalTrials.gov(NCT02275780)上进行了注册,目前尚未公开。
发现
在2014年12月1日至2015年10月20日之间,筛选了1027个人,其中769名参与者被随机分配到doravirine(n = 385)或ritonavir增强的darunavir(n = 384),两组中的383人被分配为他们分配的治疗至少一剂。大多数参与者为男性(766人中的645 [84%])和白人(560人[73%]),平均年龄为35·2岁(SD 10·6)。doravirine组的292名参与者和darunavir组的273名参与者完成了96周的治疗。在第96周时,与达那那韦组相比,更高比例的doravirine组(383中的277 [73%])达到了少于50拷贝/ mL的HIV-1 RNA浓度(383中的248 [66%];差异) 7·1%,95%CI 0·5-13·7)。无论基线特征如何,反应都相似。doravirine组383名参与者中有2名(1%)出现了对任何研究药物的治疗性耐药,而ritonavir增强型darunavir组中383名参与者中有1名(<1%)发生了治疗。在各治疗组之间,LDL胆固醇(−14·6 mg / dL,95%CI −18·2至-11·0)与非HDL胆固醇(−18·4 mg / dL)相对于基线的平均变化存在显着差异,−22·5至-14·3)。两组之间不良事件的发生频率相似。在第96周之前,由于不良事件,在停药之前没有观察到明显的治疗差异(log-rank标称p = 0·063)。最常见的不良事件(0-96周)是腹泻(doravirine组为65 [17%])在各治疗组之间,LDL胆固醇(−14·6 mg / dL,95%CI −18·2至-11·0)与非HDL胆固醇(−18·4 mg / dL)相对于基线的平均变化存在显着差异,−22·5至-14·3)。两组之间不良事件的发生频率相似。在第96周之前,由于不良事件,在停药之前没有观察到明显的治疗差异(log-rank标称p = 0·063)。最常见的不良事件(0-96周)是腹泻(doravirine组为65 [17%])在各治疗组之间,LDL胆固醇(−14·6 mg / dL,95%CI −18·2至-11·0)与非HDL胆固醇(−18·4 mg / dL)相对于基线的平均变化存在显着差异,−22·5至-14·3)。两组之间不良事件的发生频率相似。在第96周之前,由于不良事件,在停药之前没有观察到明显的治疗差异(log-rank标称p = 0·063)。最常见的不良事件(0-96周)是腹泻(doravirine组为65 [17%])VS的利托那韦升压达芦那韦组中的91 [24%]),恶心(45 [12%] VS 52 [14%]),头痛(57 [15%] VS 46 [12%]),和上呼吸道感染(51 [13%]对30 [8%])。两名参与者(每组一名)在治疗期间死亡。死亡均与研究药物无关。
解释
这些长达96周的结果支持了先前在48周时报道的doravirine的功效和安全性结果,支持使用doravirine长期治疗以前未经治疗的HIV-1感染的成年人。
资金
默克



























 京公网安备 11010802027423号
京公网安备 11010802027423号