Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
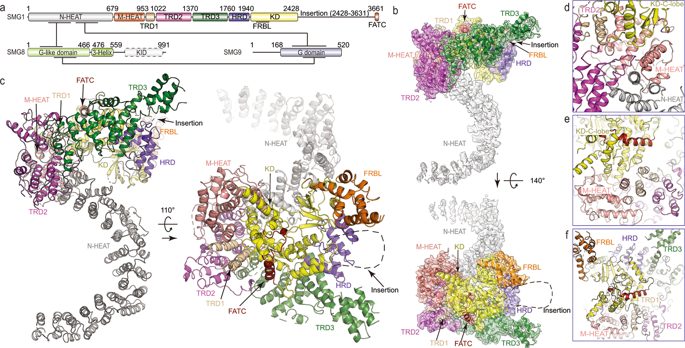
Cryo-EM structure of SMG1-SMG8-SMG9 complex.
Cell Research ( IF 44.1 ) Pub Date : 2019-11-15 , DOI: 10.1038/s41422-019-0255-3 Li Zhu 1 , Liang Li 1 , Yilun Qi 1 , Zishuo Yu 1 , Yanhui Xu 1, 2, 3, 4
Cell Research ( IF 44.1 ) Pub Date : 2019-11-15 , DOI: 10.1038/s41422-019-0255-3 Li Zhu 1 , Liang Li 1 , Yilun Qi 1 , Zishuo Yu 1 , Yanhui Xu 1, 2, 3, 4
Affiliation
|
Nonsense-mediated mRNA decay (NMD) targets premature stop codon (PTC)-containing mRNAs for rapid degradation, and is essential for mammalian embryonic development, brain development and modulation of the stress response. The key event in NMD is the SMG1-mediated phosphorylation of an RNA helicase UPF1 and SMG1 kinase activity is inhibited by SMG8 and SMG9 in an unknown mechanism. Here, we determined the cryo-EM structures of human SMG1 at 3.6 Å resolution and the SMG1-SMG8-SMG9 complex at 3.4 Å resolution, respectively. SMG8 has a C-terminal kinase inhibitory domain (KID), which covers the catalytic pocket and inhibits the kinase activity of SMG1. Structural analyses suggest that GTP hydrolysis of SMG9 would lead to a dramatic conformational change of SMG8-SMG9 and the KID would move away from the inhibitory position to restore SMG1 kinase activity. Thus, our structural and biochemical analyses provide a mechanistic understanding of SMG1-SMG8-SMG9 complex assembly and the regulatory mechanism of SMG1 kinase activity.
中文翻译:

SMG1-SMG8-SMG9 复合物的冷冻电镜结构。
无义介导的 mRNA 衰变 (NMD) 以含有提前终止密码子 (PTC) 的 mRNA 为目标进行快速降解,这对于哺乳动物胚胎发育、大脑发育和应激反应的调节至关重要。NMD 中的关键事件是 SMG1 介导的 RNA 解旋酶 UPF1 磷酸化,SMG1 激酶活性被 SMG8 和 SMG9 以未知机制抑制。在这里,我们分别以 3.6 Å 的分辨率测定了人类 SMG1 的冷冻电镜结构和分辨率为 3.4 Å 的 SMG1-SMG8-SMG9 复合物。SMG8 有一个 C 末端激酶抑制结构域 (KID),它覆盖催化口袋并抑制 SMG1 的激酶活性。结构分析表明 SMG9 的 GTP 水解会导致 SMG8-SMG9 的构象发生显着变化,并且 KID 将远离抑制位置以恢复 SMG1 激酶活性。因此,我们的结构和生化分析提供了对 SMG1-SMG8-SMG9 复合物组装和 SMG1 激酶活性调节机制的机械理解。
更新日期:2019-11-15
中文翻译:

SMG1-SMG8-SMG9 复合物的冷冻电镜结构。
无义介导的 mRNA 衰变 (NMD) 以含有提前终止密码子 (PTC) 的 mRNA 为目标进行快速降解,这对于哺乳动物胚胎发育、大脑发育和应激反应的调节至关重要。NMD 中的关键事件是 SMG1 介导的 RNA 解旋酶 UPF1 磷酸化,SMG1 激酶活性被 SMG8 和 SMG9 以未知机制抑制。在这里,我们分别以 3.6 Å 的分辨率测定了人类 SMG1 的冷冻电镜结构和分辨率为 3.4 Å 的 SMG1-SMG8-SMG9 复合物。SMG8 有一个 C 末端激酶抑制结构域 (KID),它覆盖催化口袋并抑制 SMG1 的激酶活性。结构分析表明 SMG9 的 GTP 水解会导致 SMG8-SMG9 的构象发生显着变化,并且 KID 将远离抑制位置以恢复 SMG1 激酶活性。因此,我们的结构和生化分析提供了对 SMG1-SMG8-SMG9 复合物组装和 SMG1 激酶活性调节机制的机械理解。


























 京公网安备 11010802027423号
京公网安备 11010802027423号