Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Electronic Structures and Catalytic Activities of Niobium Oxides as Electrocatalysts in Liquid‐Junction Photovoltaic Devices
Solar RRL ( IF 7.9 ) Pub Date : 2019-11-14 , DOI: 10.1002/solr.201900430 Sining Yun 1 , Yiming Si 1 , Jing Shi 2 , Taihong Zhang 1 , Yuzhi Hou 1 , Hang Liu 3 , Sheng Meng 3 , Anders Hagfeldt 4
Solar RRL ( IF 7.9 ) Pub Date : 2019-11-14 , DOI: 10.1002/solr.201900430 Sining Yun 1 , Yiming Si 1 , Jing Shi 2 , Taihong Zhang 1 , Yuzhi Hou 1 , Hang Liu 3 , Sheng Meng 3 , Anders Hagfeldt 4
Affiliation
|
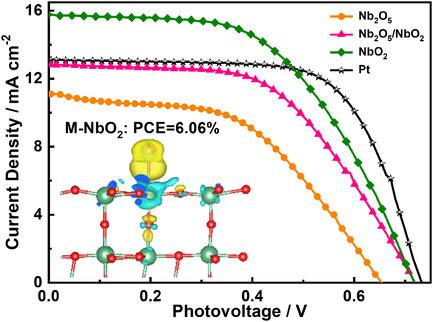
Two types of nanosized niobium oxides and their composites, pseudohexagonal Nb2O5 (TT‐Nb2O5), monoclinic NbO2 (M‐NbO2), and the coexistence of TT‐Nb2O5 and M‐NbO2 (TT‐Nb2O5/M‐NbO2), are successfully synthesized through the urea‐metal chloride route, and they exhibit excellent catalytic activity and photovoltaic performance in dye‐sensitized solar cells (DSSCs). First‐principles density function theory (DFT) calculations show that their catalytic activity is significantly influenced by their intrinsic electronic structures and properties. The lone‐pair 4d1 electrons of Nb4+ in M‐NbO2 enhance the Nb–I interaction and promote electron transfer from the M‐NbO2 counter electrode (CE) to I, thus resulting in superior catalytic properties in M‐NbO2‐based DSSCs. In addition, the adsorption energy of I on the M‐NbO2 surface is in the optimal energy range of 0.3—1.2 eV, and the Fermi level of M‐NbO2 is 0.6 eV, which is higher than the I3− reduction reaction potential, and I3− can be spontaneously reduced to 3I−. Herein, a general strategy for understanding the electronic structures and catalytic activities of transition metal compounds as CE catalysts for DSSCs is provided.
中文翻译:

液接光伏器件中氧化铌作为电催化剂的电子结构和催化活性
两种类型的纳米铌氧化物及其复合物,准六方Nb 2 O 5(TT-Nb 2 O 5),单斜NbO 2(M-NbO 2),以及TT-Nb 2 O 5和M-NbO 2(共存)TT‐Nb 2 O 5 / M‐NbO 2)是通过脲金属氯化物路线成功合成的,它们在染料敏化太阳能电池(DSSC)中表现出出色的催化活性和光伏性能。第一性原理密度函数理论(DFT)计算表明,它们的催化活性受其内在的电子结构和性质的显着影响。M‐NbO 2中Nb 4+的孤对4d 1电子增强Nb–I相互作用并促进电子从M‐NbO 2对电极(CE)转移到I,因此在M‐NbO中具有优异的催化性能基于2的DSSC。此外,I在M‐NbO 2上的吸附能表面是在0.3-1.2电子伏特的最佳能量范围,和M-NBO的费米能级2为0.6电子伏特,这比I更高3 -还原反应电位,我3 -可以自发地还原至3I - 。本文中,提供了用于理解作为DSSC的CE催化剂的过渡金属化合物的电子结构和催化活性的一般策略。
更新日期:2019-11-14
中文翻译:

液接光伏器件中氧化铌作为电催化剂的电子结构和催化活性
两种类型的纳米铌氧化物及其复合物,准六方Nb 2 O 5(TT-Nb 2 O 5),单斜NbO 2(M-NbO 2),以及TT-Nb 2 O 5和M-NbO 2(共存)TT‐Nb 2 O 5 / M‐NbO 2)是通过脲金属氯化物路线成功合成的,它们在染料敏化太阳能电池(DSSC)中表现出出色的催化活性和光伏性能。第一性原理密度函数理论(DFT)计算表明,它们的催化活性受其内在的电子结构和性质的显着影响。M‐NbO 2中Nb 4+的孤对4d 1电子增强Nb–I相互作用并促进电子从M‐NbO 2对电极(CE)转移到I,因此在M‐NbO中具有优异的催化性能基于2的DSSC。此外,I在M‐NbO 2上的吸附能表面是在0.3-1.2电子伏特的最佳能量范围,和M-NBO的费米能级2为0.6电子伏特,这比I更高3 -还原反应电位,我3 -可以自发地还原至3I - 。本文中,提供了用于理解作为DSSC的CE催化剂的过渡金属化合物的电子结构和催化活性的一般策略。


























 京公网安备 11010802027423号
京公网安备 11010802027423号