当前位置:
X-MOL 学术
›
Biochemistry
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Role of Glycosyltransferase 25 Domain 1 in Type I Collagen Glycosylation and Molecular Phenotypes.
Biochemistry ( IF 2.9 ) Pub Date : 2019-12-05 , DOI: 10.1021/acs.biochem.8b00984 Masahiko Terajima 1 , Yuki Taga 2 , Marnisa Sricholpech 3 , Yukako Kayashima 4 , Noriko Sumida 1 , Nobuyo Maeda 4 , Shunji Hattori 2 , Mitsuo Yamauchi 1
Biochemistry ( IF 2.9 ) Pub Date : 2019-12-05 , DOI: 10.1021/acs.biochem.8b00984 Masahiko Terajima 1 , Yuki Taga 2 , Marnisa Sricholpech 3 , Yukako Kayashima 4 , Noriko Sumida 1 , Nobuyo Maeda 4 , Shunji Hattori 2 , Mitsuo Yamauchi 1
Affiliation
|
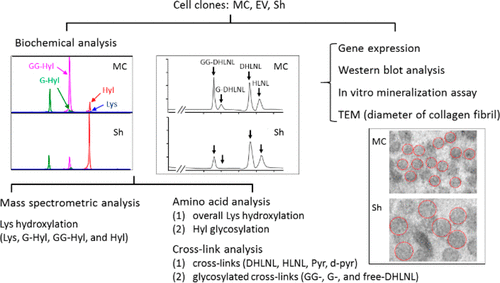
Glycosylation in type I collagen occurs as O-linked galactosyl- (G-) lesser and glucosylgalactosyl-hydroxylysine (GG-Hyl); however, its biological significance is still not well understood. To investigate the function of this modification in bone, we have generated preosteoblast MC3T3-E1 (MC)-derived clones, short hairpin (Sh) clones, in which Glt25d1 gene expression was stably suppressed. In Sh clones, the GLT25D1 protein levels were markedly diminished in comparison to controls (MC and those transfected with the empty vector). In Sh collagen, levels of both G- and GG-Hyl were significantly diminished with a concomitant increase in the level of free-Hyl. In addition, the level of immature divalent cross-links significantly diminished while the level of the mature trivalent cross-link increased. As determined by mass spectrometric analysis, seven glycosylation sites were identified in type I collagen and the most predominant site was at the helical cross-linking site, α1-87. At all of the glycosylation sites, the relative levels of G- and GG-Hyl were markedly diminished, i.e., by ∼50-75%, in Sh collagen, and at five of these sites, the level of Lys hydroxylation was significantly increased. The collagen fibrils in Sh clones were larger, and mineralization was impaired. These results indicate that GLT25D1 catalyzes galactosylation of Hyl throughout the type I collagen molecule and that this modification may regulate maturation of collagen cross-linking, fibrillogenesis, and mineralization.
中文翻译:

糖基转移酶25结构域1在I型胶原糖基化和分子表型中的作用。
I型胶原的糖基化作用以较少的O-连接半乳糖基(G-)和葡萄糖基半乳糖基-羟基赖氨酸(GG-Hyl)的形式出现;然而,其生物学意义仍未得到很好的理解。为了研究这种修饰在骨中的功能,我们已经生成了成骨细胞MC3T3-E1(MC)衍生的克隆,短发夹(Sh)克隆,其中Glt25d1基因的表达被稳定地抑制了。在Sh克隆中,与对照(MC和用空载体转染的对照)相比,GLT25D1蛋白水平显着降低。在Sh胶原蛋白中,G-和GG-Hyl的水平均显着降低,同时游离Hyl的水平也随之增加。另外,未成熟的二价交联的水平显着降低,而成熟的三价交联的水平增加。通过质谱分析确定 在I型胶原蛋白中鉴定出七个糖基化位点,最主要的位点位于螺旋交联位点α1-87。在所有糖基化位点上,Sh胶原蛋白的G-和GG-Hyl相对水平显着降低,即降低了约50-75%,并且在这些位点中的五个位点,Lys羟化水平显着提高。Sh克隆中的胶原蛋白原纤维较大,并且矿化受损。这些结果表明,GLT25D1会在整个I型胶原分子中催化Hyl的半乳糖基化,并且这种修饰可能会调节胶原交联,原纤维形成和矿化的成熟。在Sh胶原蛋白中,G-和GG-Hyl的相对水平显着降低,即降低了约50-75%,并且在这五个位置中,Lys的羟化水平显着提高。Sh克隆中的胶原蛋白原纤维较大,并且矿化受损。这些结果表明,GLT25D1会在整个I型胶原分子中催化Hyl的半乳糖基化,并且这种修饰可能会调节胶原交联,原纤维形成和矿化的成熟。在Sh胶原蛋白中,G-和GG-Hyl的相对水平显着降低,即降低了约50-75%,并且在这些位点中的五个位点,Lys羟化水平显着提高。Sh克隆中的胶原蛋白原纤维较大,并且矿化受损。这些结果表明,GLT25D1会在整个I型胶原分子中催化Hyl的半乳糖基化,并且这种修饰可能会调节胶原交联,原纤维形成和矿化的成熟。
更新日期:2019-12-06
中文翻译:

糖基转移酶25结构域1在I型胶原糖基化和分子表型中的作用。
I型胶原的糖基化作用以较少的O-连接半乳糖基(G-)和葡萄糖基半乳糖基-羟基赖氨酸(GG-Hyl)的形式出现;然而,其生物学意义仍未得到很好的理解。为了研究这种修饰在骨中的功能,我们已经生成了成骨细胞MC3T3-E1(MC)衍生的克隆,短发夹(Sh)克隆,其中Glt25d1基因的表达被稳定地抑制了。在Sh克隆中,与对照(MC和用空载体转染的对照)相比,GLT25D1蛋白水平显着降低。在Sh胶原蛋白中,G-和GG-Hyl的水平均显着降低,同时游离Hyl的水平也随之增加。另外,未成熟的二价交联的水平显着降低,而成熟的三价交联的水平增加。通过质谱分析确定 在I型胶原蛋白中鉴定出七个糖基化位点,最主要的位点位于螺旋交联位点α1-87。在所有糖基化位点上,Sh胶原蛋白的G-和GG-Hyl相对水平显着降低,即降低了约50-75%,并且在这些位点中的五个位点,Lys羟化水平显着提高。Sh克隆中的胶原蛋白原纤维较大,并且矿化受损。这些结果表明,GLT25D1会在整个I型胶原分子中催化Hyl的半乳糖基化,并且这种修饰可能会调节胶原交联,原纤维形成和矿化的成熟。在Sh胶原蛋白中,G-和GG-Hyl的相对水平显着降低,即降低了约50-75%,并且在这五个位置中,Lys的羟化水平显着提高。Sh克隆中的胶原蛋白原纤维较大,并且矿化受损。这些结果表明,GLT25D1会在整个I型胶原分子中催化Hyl的半乳糖基化,并且这种修饰可能会调节胶原交联,原纤维形成和矿化的成熟。在Sh胶原蛋白中,G-和GG-Hyl的相对水平显着降低,即降低了约50-75%,并且在这些位点中的五个位点,Lys羟化水平显着提高。Sh克隆中的胶原蛋白原纤维较大,并且矿化受损。这些结果表明,GLT25D1会在整个I型胶原分子中催化Hyl的半乳糖基化,并且这种修饰可能会调节胶原交联,原纤维形成和矿化的成熟。



























 京公网安备 11010802027423号
京公网安备 11010802027423号