Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Cryo-EM Structure of Nucleotide-Bound Tel1ATM Unravels the Molecular Basis of Inhibition and Structural Rationale for Disease-Associated Mutations.
Structure ( IF 5.7 ) Pub Date : 2019-11-04 , DOI: 10.1016/j.str.2019.10.012 Luke A Yates 1 , Rhys M Williams 1 , Sarem Hailemariam 2 , Rafael Ayala 1 , Peter Burgers 2 , Xiaodong Zhang 1
Structure ( IF 5.7 ) Pub Date : 2019-11-04 , DOI: 10.1016/j.str.2019.10.012 Luke A Yates 1 , Rhys M Williams 1 , Sarem Hailemariam 2 , Rafael Ayala 1 , Peter Burgers 2 , Xiaodong Zhang 1
Affiliation
|
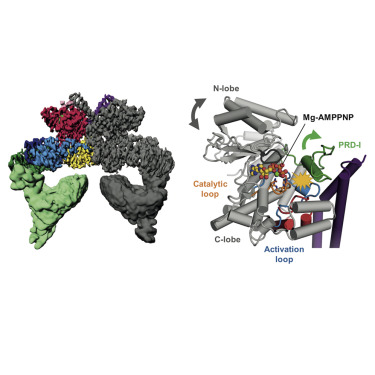
Yeast Tel1 and its highly conserved human ortholog ataxia-telangiectasia mutated (ATM) are large protein kinases central to the maintenance of genome integrity. Mutations in ATM are found in ataxia-telangiectasia (A-T) patients and ATM is one of the most frequently mutated genes in many cancers. Using cryoelectron microscopy, we present the structure of Tel1 in a nucleotide-bound state. Our structure reveals molecular details of key residues surrounding the nucleotide binding site and provides a structural and molecular basis for its intrinsically low basal activity. We show that the catalytic residues are in a productive conformation for catalysis, but the phosphatidylinositol 3-kinase-related kinase (PIKK) regulatory domain insert restricts peptide substrate access and the N-lobe is in an open conformation, thus explaining the requirement for Tel1 activation. Structural comparisons with other PIKKs suggest a conserved and common allosteric activation mechanism. Our work also provides a structural rationale for many mutations found in A-T and cancer.
中文翻译:

核苷酸结合的Tel1ATM的低温EM结构揭示了疾病相关突变的抑制分子基础和结构原理。
酵母Tel1及其高度保守的人类直系同源性共济失调毛细血管扩张(ATM)是维持基因组完整性至关重要的大型蛋白激酶。在共济失调毛细血管扩张症(AT)患者中发现了ATM突变,而ATM是许多癌症中最常见的突变基因之一。使用冷冻电子显微镜,我们目前处于核苷酸结合状态的Tel1的结构。我们的结构揭示了核苷酸结合位点周围关键残基的分子细节,并为其固有的低基础活性提供了结构和分子基础。我们显示催化残基处于催化的生产构型中,但磷脂酰肌醇3激酶相关激酶(PIKK)调节域插入片段限制了肽底物的进入,而N瓣处于开放构型,因此,说明了激活Tel1的要求。与其他PIKK的结构比较表明,保守的和常见的变构激活机制。我们的工作还为AT和癌症中发现的许多突变提供了结构原理。
更新日期:2019-11-15
中文翻译:

核苷酸结合的Tel1ATM的低温EM结构揭示了疾病相关突变的抑制分子基础和结构原理。
酵母Tel1及其高度保守的人类直系同源性共济失调毛细血管扩张(ATM)是维持基因组完整性至关重要的大型蛋白激酶。在共济失调毛细血管扩张症(AT)患者中发现了ATM突变,而ATM是许多癌症中最常见的突变基因之一。使用冷冻电子显微镜,我们目前处于核苷酸结合状态的Tel1的结构。我们的结构揭示了核苷酸结合位点周围关键残基的分子细节,并为其固有的低基础活性提供了结构和分子基础。我们显示催化残基处于催化的生产构型中,但磷脂酰肌醇3激酶相关激酶(PIKK)调节域插入片段限制了肽底物的进入,而N瓣处于开放构型,因此,说明了激活Tel1的要求。与其他PIKK的结构比较表明,保守的和常见的变构激活机制。我们的工作还为AT和癌症中发现的许多突变提供了结构原理。


























 京公网安备 11010802027423号
京公网安备 11010802027423号