当前位置:
X-MOL 学术
›
Exp. Neurol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Inhibition of excessive kallikrein-8 improves neuroplasticity in Alzheimer's disease mouse model.
Experimental Neurology ( IF 5.3 ) Pub Date : 2019-11-14 , DOI: 10.1016/j.expneurol.2019.113115 Yvonne Münster 1 , Kathy Keyvani 1 , Arne Herring 1
Experimental Neurology ( IF 5.3 ) Pub Date : 2019-11-14 , DOI: 10.1016/j.expneurol.2019.113115 Yvonne Münster 1 , Kathy Keyvani 1 , Arne Herring 1
Affiliation
|
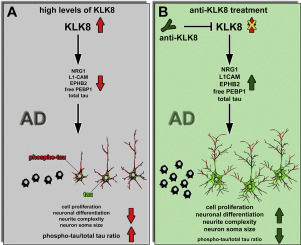
We recently identified excessive cerebral kallikrein-8 (KLK8) mRNA and protein levels at incipient stages of Alzheimer's disease (AD) in AD patients and TgCRND8 mice. Additionally, we showed that antibody-mediated KLK8 inhibition exerts therapeutic effects on AD along with enhancing neuroplasticity, resulting in improved spatial memory in mice. Mounting evidence further substantiates an important role of the protease KLK8 in neuroplasticity. In the present study we sought to gain new mechanistic insights in the interplay between KLK8, neuroplasticity and tau phosphorylation in the context of AD. We here demonstrate that KLK8 inhibition increased the number of hippocampal Ki-67 and doublecortin positive, proliferative neuronal progenitor cells in transgenic mice, whereas the same action in wildtypes had no effect. In line with these results, KLK8 inhibition reduced the levels of its pro-proliferative interaction partners KLK6 and protease-activated receptor 2 only in wildtypes, while the levels of its proliferation-supporting substrate neuregulin-1 and the non-complexed form of its complexing-partner phosphatidylethanolamine binding protein 1 were enhanced in both genotypes. Concomitant incubation of beta-amyloid (Aβ)-producing primary neurons with KLK8 and its inhibitory antibody increased neurite complexity and soma size. KLK8 inhibition in SH-SY5Y cells or in primary neurons increased levels of the neuroplasticity-supporting KLK8 substrate ephrin receptor B2 (EPHB2) and total tau while decreasing the relative amount of phospho-tau in relation to total tau. KLK8 blockade further enhanced cell proliferation in SH-SY5Y cells. Additional co-incubation with an inhibitory anti-EPHB2 antibody decreased total tau levels and neurite complexity and increased the ratio of phospho-tau/total tau, underlining the key role of EPHB2 on this plastic change. In a reverse in vitro approach, KLK8 induction reduced EPHB2 and total tau and increased the ratio of phospho-tau/total tau, leading to impaired proliferation and neuronal differentiation. These results underline the therapeutic potential of KLK8 inhibition by counteracting plasticity deficits in AD-affected brain.
中文翻译:

抑制激肽释放酶-8的过量表达可改善阿尔茨海默氏病小鼠模型的神经可塑性。
我们最近在AD患者和TgCRND8小鼠的阿尔茨海默氏病(AD)初始阶段发现了过量的脑激肽释放酶8(KLK8)mRNA和蛋白水平。此外,我们表明抗体介导的KLK8抑制作用对AD产生治疗作用,同时增强神经可塑性,从而改善了小鼠的空间记忆。越来越多的证据进一步证实了蛋白酶KLK8在神经可塑性中的重要作用。在本研究中,我们试图获得有关AD背景下KLK8,神经可塑性和tau磷酸化之间相互作用的新机制的见解。我们在这里证明了KLK8抑制作用在转基因小鼠中增加了海马Ki-67和双皮质素阳性,增生性神经元祖细胞的数量,而在野生型中相同的作用没有作用。根据这些结果,KLK8抑制仅在野生型中降低其促增殖相互作用伴侣KLK6和蛋白酶激活受体2的水平,而其增殖支持底物神经调节蛋白1的水平及其复合伴侣磷脂酰乙醇胺结合蛋白1的非复合形式的水平。在两种基因型中均得到增强。与KLK8及其抑制性抗体同时孵育产生β-淀粉样蛋白(Aβ)的原代神经元会增加神经突的复杂性和躯体大小。SH-SY5Y细胞或原代神经元中的KLK8抑制增加了支持神经可塑性的KLK8底物ephrin受体B2(EPHB2)和总tau的水平,同时降低了相对于总tau的磷酸化tau的相对量。KLK8阻断进一步增强了SH-SY5Y细胞中的细胞增殖。与抑制性抗EPHB2抗体进行的额外共孵育降低了总tau含量和神经突复杂性,并增加了磷酸化tau /总tau的比例,从而强调了EPHB2在这种塑性变化中的关键作用。在反向体外方法中,KLK8诱导降低了EPHB2和总tau,并增加了磷酸化tau /总tau的比例,从而导致增殖和神经元分化受损。这些结果通过抵消受AD影响的大脑中的可塑性缺陷,突显了KLK8抑制的治疗潜力。导致受损的增殖和神经元分化。这些结果通过抵消受AD影响的大脑中的可塑性缺陷,突显了KLK8抑制的治疗潜力。导致受损的增殖和神经元分化。这些结果通过抵消受AD影响的大脑中的可塑性缺陷,突显了KLK8抑制的治疗潜力。
更新日期:2019-11-14
中文翻译:

抑制激肽释放酶-8的过量表达可改善阿尔茨海默氏病小鼠模型的神经可塑性。
我们最近在AD患者和TgCRND8小鼠的阿尔茨海默氏病(AD)初始阶段发现了过量的脑激肽释放酶8(KLK8)mRNA和蛋白水平。此外,我们表明抗体介导的KLK8抑制作用对AD产生治疗作用,同时增强神经可塑性,从而改善了小鼠的空间记忆。越来越多的证据进一步证实了蛋白酶KLK8在神经可塑性中的重要作用。在本研究中,我们试图获得有关AD背景下KLK8,神经可塑性和tau磷酸化之间相互作用的新机制的见解。我们在这里证明了KLK8抑制作用在转基因小鼠中增加了海马Ki-67和双皮质素阳性,增生性神经元祖细胞的数量,而在野生型中相同的作用没有作用。根据这些结果,KLK8抑制仅在野生型中降低其促增殖相互作用伴侣KLK6和蛋白酶激活受体2的水平,而其增殖支持底物神经调节蛋白1的水平及其复合伴侣磷脂酰乙醇胺结合蛋白1的非复合形式的水平。在两种基因型中均得到增强。与KLK8及其抑制性抗体同时孵育产生β-淀粉样蛋白(Aβ)的原代神经元会增加神经突的复杂性和躯体大小。SH-SY5Y细胞或原代神经元中的KLK8抑制增加了支持神经可塑性的KLK8底物ephrin受体B2(EPHB2)和总tau的水平,同时降低了相对于总tau的磷酸化tau的相对量。KLK8阻断进一步增强了SH-SY5Y细胞中的细胞增殖。与抑制性抗EPHB2抗体进行的额外共孵育降低了总tau含量和神经突复杂性,并增加了磷酸化tau /总tau的比例,从而强调了EPHB2在这种塑性变化中的关键作用。在反向体外方法中,KLK8诱导降低了EPHB2和总tau,并增加了磷酸化tau /总tau的比例,从而导致增殖和神经元分化受损。这些结果通过抵消受AD影响的大脑中的可塑性缺陷,突显了KLK8抑制的治疗潜力。导致受损的增殖和神经元分化。这些结果通过抵消受AD影响的大脑中的可塑性缺陷,突显了KLK8抑制的治疗潜力。导致受损的增殖和神经元分化。这些结果通过抵消受AD影响的大脑中的可塑性缺陷,突显了KLK8抑制的治疗潜力。



























 京公网安备 11010802027423号
京公网安备 11010802027423号