Bone Research ( IF 12.7 ) Pub Date : 2019-11-14 , DOI: 10.1038/s41413-019-0076-5 Michaela Tencerova 1 , Elizabeth Rendina-Ruedy 2 , Ditte Neess 3 , Nils Færgeman 3 , Florence Figeac 1 , Dalia Ali 1 , Morten Danielsen 4 , Anders Haakonsson 1 , Clifford J Rosen 2 , Moustapha Kassem 1, 5
|
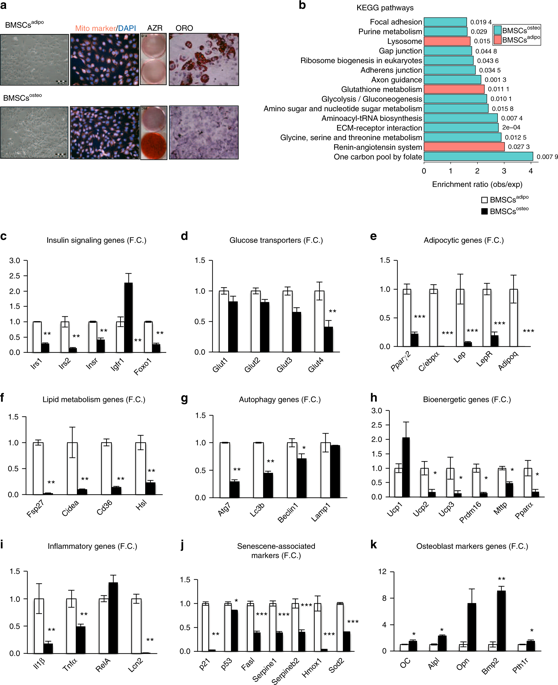
Enhanced bone marrow adipogenesis and impaired osteoblastogenesis have been observed in obesity, suggesting that the metabolic microenvironment regulates bone marrow adipocyte and osteoblast progenitor differentiation fate. To determine the molecular mechanisms, we studied two immortalized murine cell lines of adipocyte or osteoblast progenitors (BMSCsadipo and BMSCsosteo, respectively) under basal and adipogenic culture conditions. At baseline, BMSCsadipo, and BMSCsosteo exhibit a distinct metabolic program evidenced by the presence of specific global gene expression, cellular bioenergetics, and metabolomic signatures that are dependent on insulin signaling and glycolysis in BMSCsosteo versus oxidative phosphorylation in BMSCsadipo. To test the flexibility of the metabolic program, we treated BMSCsadipo with parathyroid hormone, S961 (an inhibitor of insulin signaling) and oligomycin (an inhibitor of oxidative phosphorylation). The treatment induced significant changes in cellular bioenergetics that were associated with decreased adipocytic differentiation. Similarly, 12 weeks of a high-fat diet in mice led to the expansion of adipocyte progenitors, enhanced adipocyte differentiation and insulin signaling in cultured BMSCs. Our data demonstrate that BMSC progenitors possess a distinct metabolic program and are poised to respond to exogenous metabolic cues that regulate their differentiation fate.
中文翻译:

代谢编程决定了小鼠骨髓基质祖细胞的谱系分化命运。
在肥胖症中观察到增强的骨髓脂肪生成和受损的成骨细胞生成,表明代谢微环境调节骨髓脂肪细胞和成骨细胞祖细胞分化命运。为了确定分子机制,我们在基础和成脂培养条件下研究了脂肪细胞或成骨细胞祖细胞的两种永生化小鼠细胞系(分别为 BMSCs adipo和 BMSCs osteo )。在基线时,BMSCs adipo和 BMSCs osteo表现出独特的代谢程序,具体证据是存在特定的全局基因表达、细胞生物能量学和代谢组学特征,这些特征依赖于 BMSCs osteo中的胰岛素信号和糖酵解与 BMSCs 脂肪中的氧化磷酸化。为了测试代谢程序的灵活性,我们用甲状旁腺激素、 S961(胰岛素信号抑制剂)和寡霉素(氧化磷酸化抑制剂)处理脂肪骨髓间充质干细胞。该治疗引起与脂肪细胞分化减少相关的细胞生物能量学的显着变化。同样,在小鼠中进行 12 周的高脂肪饮食导致脂肪细胞祖细胞扩增,增强脂肪细胞分化和培养的 BMSC 中的胰岛素信号传导。我们的数据表明 BMSC 祖细胞拥有独特的代谢程序,并准备好响应调节其分化命运的外源性代谢线索。



























 京公网安备 11010802027423号
京公网安备 11010802027423号