当前位置:
X-MOL 学术
›
BBA Gen. Subj.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Unveiling the structural features of nonnative trimers of human superoxide dismutase 1.
Biochimica et Biophysica Acta (BBA) - General Subjects ( IF 3 ) Pub Date : 2019-11-14 , DOI: 10.1016/j.bbagen.2019.129483 Wei-Chih Chao , Jyh-Feng Lu , Jinn-Shyan Wang , Tzu-Hsuan Chiang , Li-Ju Lin , Yao-Lin Lee , Pi-Tai Chou
Biochimica et Biophysica Acta (BBA) - General Subjects ( IF 3 ) Pub Date : 2019-11-14 , DOI: 10.1016/j.bbagen.2019.129483 Wei-Chih Chao , Jyh-Feng Lu , Jinn-Shyan Wang , Tzu-Hsuan Chiang , Li-Ju Lin , Yao-Lin Lee , Pi-Tai Chou
|
BACKGROUND
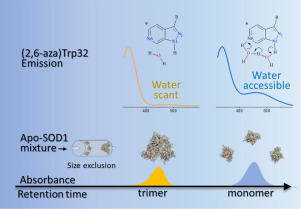
Human SOD1 contains a single tryptophan residue (W32) which has been identified as a site of oxidative modification and a potentiator of aggregation involving in familial amyotrophic lateral sclerosis (fALS). In situ substitution of a tryptophan analog, 2,6-diazatryptophan ((2,6-aza)Trp) with its unique water-catalyzed proton transfer property, into proteins exhibits extraordinary sensitivity in the detection of subtle water-associated structural changes with only a few micro-molar concentration of samples.
METHODS
A combination of size-exclusion chromatography and water-catalyzed fluorescent emission was utilized to probe the structural features of metastable SOD1 nonnative trimers, the potential neurotoxic species in the fALS.
RESULTS
The monomer of apo-A4V SOD1 exhibits variable conformations and the fastest trimeric formation rate compared to that of wild type and I113T. The trimeric A4V SOD1 exhibits the least water molecules surrounding the W32, while I113T and the wild type appear to have more water molecules in the proximity of W32. A small molecule stabilizer, 5-fluorouridine, effects the structural conformation of SOD1 nonnative trimers.
CONCLUSIONS
Our studies unveil new insights into water-associated structural changes of SOD1 nonnative trimers and demonstrate that in situ incorporation of (2,6-aza)Trp is a sensitive and powerful tool for probing subtle changes of water environments during protein aggregation.
GENERAL SIGNIFICANCE
The water-sensitive probe, (2,6-aza)Trp, demonstrates superior sensitivity for detecting modulation of water microsolvation, structural conformation during oligomer formation and 5FUrd binding to both wild type and mutant SOD1.
中文翻译:

揭示人类超氧化物歧化酶1的非天然三聚体的结构特征。
背景技术人SOD1包含单个色氨酸残基(W32),其已被鉴定为涉及家族性肌萎缩性侧索硬化症(fALS)的氧化修饰位点和聚集增强剂。用其独特的水催化质子转移特性将色氨酸类似物2,6-二氮杂色氨酸((2,6-aza)Trp)原位取代为蛋白质,在检测与水有关的细微结构变化方面显示出非凡的灵敏度几微摩尔浓度的样品。方法采用体积排阻色谱法和水催化荧光发射法相结合的方法,检测亚稳态SOD1非天然三聚体(fALS中潜在的神经毒性物质)的结构特征。结果与野生型和I113T相比,apo-A4V SOD1的单体具有可变的构象和最快的三聚体形成速率。三聚体A4V SOD1在W32周围表现出最少的水分子,而I113T和野生型在W32附近表现出更多的水分子。小分子稳定剂5-氟尿苷影响SOD1非天然三聚体的结构构象。结论我们的研究揭示了对SOD1非天然三聚体与水相关的结构变化的新见解,并证明了(2,6-aza)Trp的原位掺入是探测蛋白质聚集过程中水环境细微变化的灵敏且功能强大的工具。一般意义水敏探针(2,6-aza)Trp表现出优异的灵敏度,可检测水微溶剂化的调节,
更新日期:2019-11-14
中文翻译:

揭示人类超氧化物歧化酶1的非天然三聚体的结构特征。
背景技术人SOD1包含单个色氨酸残基(W32),其已被鉴定为涉及家族性肌萎缩性侧索硬化症(fALS)的氧化修饰位点和聚集增强剂。用其独特的水催化质子转移特性将色氨酸类似物2,6-二氮杂色氨酸((2,6-aza)Trp)原位取代为蛋白质,在检测与水有关的细微结构变化方面显示出非凡的灵敏度几微摩尔浓度的样品。方法采用体积排阻色谱法和水催化荧光发射法相结合的方法,检测亚稳态SOD1非天然三聚体(fALS中潜在的神经毒性物质)的结构特征。结果与野生型和I113T相比,apo-A4V SOD1的单体具有可变的构象和最快的三聚体形成速率。三聚体A4V SOD1在W32周围表现出最少的水分子,而I113T和野生型在W32附近表现出更多的水分子。小分子稳定剂5-氟尿苷影响SOD1非天然三聚体的结构构象。结论我们的研究揭示了对SOD1非天然三聚体与水相关的结构变化的新见解,并证明了(2,6-aza)Trp的原位掺入是探测蛋白质聚集过程中水环境细微变化的灵敏且功能强大的工具。一般意义水敏探针(2,6-aza)Trp表现出优异的灵敏度,可检测水微溶剂化的调节,



























 京公网安备 11010802027423号
京公网安备 11010802027423号