当前位置:
X-MOL 学术
›
Regul. Toxicol. Pharmacol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Qualification of impurities based on metabolite data.
Regulatory Toxicology and Pharmacology ( IF 3.4 ) Pub Date : 2019-11-14 , DOI: 10.1016/j.yrtph.2019.104524 Lars Weidolf 1 , Thomas Andersson 2 , Joel P Bercu 3 , Andreas Brink 4 , Susanne Glowienke 5 , James Harvey 6 , Martin A Hayes 7 , Pascale Jacques 8 , Chuang Lu 9 , Nenad Manevski 10 , Wolfgang Muster 4 , Raphael Nudelman 11 , Ron Ogilvie 12 , Jenny Ottosson 13 , Andrew Teasdale 14 , Bruce Trela 15
Regulatory Toxicology and Pharmacology ( IF 3.4 ) Pub Date : 2019-11-14 , DOI: 10.1016/j.yrtph.2019.104524 Lars Weidolf 1 , Thomas Andersson 2 , Joel P Bercu 3 , Andreas Brink 4 , Susanne Glowienke 5 , James Harvey 6 , Martin A Hayes 7 , Pascale Jacques 8 , Chuang Lu 9 , Nenad Manevski 10 , Wolfgang Muster 4 , Raphael Nudelman 11 , Ron Ogilvie 12 , Jenny Ottosson 13 , Andrew Teasdale 14 , Bruce Trela 15
Affiliation
|
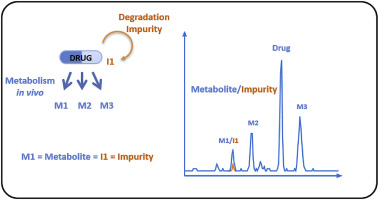
Regulatory Guidance documents ICH Q3A (R2) and ICH Q3B (R2) state that "impurities that are also significant metabolites present in animal and/or human studies are generally considered qualified". However, no guidance is provided regarding data requirements for qualification, nor is a definition of the term "significant metabolite" provided. An opportunity is provided to define those categories and potentially avoid separate toxicity studies to qualify impurities. This can reduce cost, animal use and time, and avoid delays in drug development progression. If the concentration or amount of a metabolite, in animals or human, is similar to that of the known, structurally identical impurity (arising from the administered test material), the qualification of the impurity on the grounds of it also being a metabolite is justified. We propose two complementary approaches to support conclusions to this effect: 1) demonstrate that the impurity is formed by metabolism in animals and/or man, based preferably on plasma exposures or, alternatively, amounts excreted in urine, and, where appropriate, 2) show that animal exposure to (or amount of) the impurity/metabolite is equal or greater in animals than in humans. An important factor of both assessments is the maximum theoretical concentration (or amount) (MTC or MTA) of the impurity/metabolite achievable from the administered dose and recommendations on the estimation of the MTC and MTA are presented.
中文翻译:

根据代谢物数据对杂质进行鉴定。
监管指南文件ICH Q3A(R2)和ICH Q3B(R2)指出,“杂质也是动物和/或人体研究中存在的重要代谢产物,通常被认为是合格的”。但是,未提供有关鉴定数据要求的指南,也未提供术语“重要代谢物”的定义。提供了一个机会来定义这些类别,并有可能避免进行单独的毒性研究以鉴定杂质。这样可以减少成本,减少动物使用和时间,并避免药物开发进度的延迟。如果在动物或人类中代谢物的浓度或量与已知的结构相同的杂质(源自所施用的测试物质)的浓度或量相似,则以该杂质也为代谢物为由鉴定该杂质是合理的。我们提出了两种补充方法来支持对此效果的结论:1)证明杂质是由动物和/或人的新陈代谢形成的,优选地基于血浆暴露或尿中排泄的量,以及(如适用)2)表明动物对动物的杂质/代谢物暴露(或含量)等于或大于人类。两种评估的重要因素是可从施用剂量中获得的杂质/代谢物的最大理论浓度(或含量)(MTC或MTA),并提出了有关MTC和MTA估算的建议。尿液中的排泄量,并在适当的情况下2)表明动物与人相比,动物对杂质/代谢物的暴露量(或含量)等于或大于人。两种评估的重要因素是可从施用剂量中获得的杂质/代谢物的最大理论浓度(或含量)(MTC或MTA),并提出了有关MTC和MTA估算的建议。尿液中的排泄量,并在适当的情况下2)表明动物与人相比,动物对杂质/代谢物的暴露量(或含量)等于或大于人。两种评估的重要因素是可从施用剂量中获得的杂质/代谢物的最大理论浓度(或含量)(MTC或MTA),并提出了有关MTC和MTA估算的建议。
更新日期:2019-11-14
中文翻译:

根据代谢物数据对杂质进行鉴定。
监管指南文件ICH Q3A(R2)和ICH Q3B(R2)指出,“杂质也是动物和/或人体研究中存在的重要代谢产物,通常被认为是合格的”。但是,未提供有关鉴定数据要求的指南,也未提供术语“重要代谢物”的定义。提供了一个机会来定义这些类别,并有可能避免进行单独的毒性研究以鉴定杂质。这样可以减少成本,减少动物使用和时间,并避免药物开发进度的延迟。如果在动物或人类中代谢物的浓度或量与已知的结构相同的杂质(源自所施用的测试物质)的浓度或量相似,则以该杂质也为代谢物为由鉴定该杂质是合理的。我们提出了两种补充方法来支持对此效果的结论:1)证明杂质是由动物和/或人的新陈代谢形成的,优选地基于血浆暴露或尿中排泄的量,以及(如适用)2)表明动物对动物的杂质/代谢物暴露(或含量)等于或大于人类。两种评估的重要因素是可从施用剂量中获得的杂质/代谢物的最大理论浓度(或含量)(MTC或MTA),并提出了有关MTC和MTA估算的建议。尿液中的排泄量,并在适当的情况下2)表明动物与人相比,动物对杂质/代谢物的暴露量(或含量)等于或大于人。两种评估的重要因素是可从施用剂量中获得的杂质/代谢物的最大理论浓度(或含量)(MTC或MTA),并提出了有关MTC和MTA估算的建议。尿液中的排泄量,并在适当的情况下2)表明动物与人相比,动物对杂质/代谢物的暴露量(或含量)等于或大于人。两种评估的重要因素是可从施用剂量中获得的杂质/代谢物的最大理论浓度(或含量)(MTC或MTA),并提出了有关MTC和MTA估算的建议。


























 京公网安备 11010802027423号
京公网安备 11010802027423号