当前位置:
X-MOL 学术
›
Toxicol. Appl. Pharmacol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Ginsenoside compound K alleviates sodium valproate-induced hepatotoxicity in rats via antioxidant effect, regulation of peroxisome pathway and iron homeostasis.
Toxicology and Applied Pharmacology ( IF 3.8 ) Pub Date : 2019-11-14 , DOI: 10.1016/j.taap.2019.114829 Luping Zhou 1 , Lulu Chen 2 , Xiangchang Zeng 1 , Jianwei Liao 1 , Dongsheng Ouyang 2
Toxicology and Applied Pharmacology ( IF 3.8 ) Pub Date : 2019-11-14 , DOI: 10.1016/j.taap.2019.114829 Luping Zhou 1 , Lulu Chen 2 , Xiangchang Zeng 1 , Jianwei Liao 1 , Dongsheng Ouyang 2
Affiliation
|
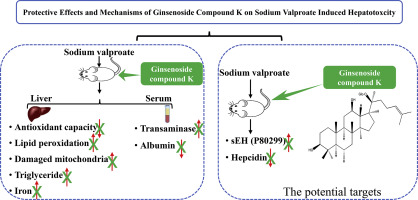
Sodium valproate (SVP) is a first-line treatment for various forms of epilepsy; however, it can cause severe liver injury. Ginsenoside compound K (G-CK) is the main active ingredient of the traditional herbal medicine ginseng. According to our previous research, SVP-induced elevation of ALT and AST levels, as well as pathological changes of liver tissue, was believed to be significantly reversed by G-CK in LiCl-pilocarpine induced epileptic rats. Thus, we aimed to evaluate the protective effect of G-CK on hepatotoxicity caused by SVP. The rats treated with SVP showed liver injury with evident increases in hepatic index, transaminases activity, alkaline phosphatase level, hepatic triglyceride and lipid peroxidation; significant decreases in plasma albumin level and antioxidant capacity; and obvious changes in histopathological and subcellular structures. All of these changes could be mitigated by co-administration with G-CK. Proteomic analysis indicated that hepcidin, soluble epoxide hydrolase (sEH, UniProt ID P80299), and the peroxisome pathway were involved in the hepatoprotective effect of G-CK. Changes in protein expression of hepcidin and sEH were verified by ELISA and Western blot analysis, respectively. In addition, we observed that the hepatic iron rose in SVP group and decreased in the combination group. In summary, our findings demonstrate the clear hepatoprotective effect of G-CK against SVP-induced hepatotoxicity through the antioxidant effect, regulation of peroxisome pathway relying on sEH (P80299) downregulation, as well as regulation of iron homeostasis dependent on hepcidin upregulation.
中文翻译:

人参皂甙化合物K通过抗氧化作用,调节过氧化物酶体途径和铁稳态来减轻丙戊酸钠诱导的大鼠肝毒性。
丙戊酸钠(SVP)是治疗各种形式癫痫的一线治疗药物。但是,它可能导致严重的肝损伤。人参皂苷化合物K(G-CK)是传统草药人参的主要活性成分。根据我们先前的研究,据信G-CK可在LiCl-毛果芸香碱诱发的癫痫大鼠中显着逆转SVP诱导的ALT和AST水平升高以及肝脏组织的病理变化。因此,我们旨在评估G-CK对SVP引起的肝毒性的保护作用。用SVP治疗的大鼠表现出肝损伤,肝指数,转氨酶活性,碱性磷酸酶水平,肝甘油三酸酯和脂质过氧化明显增加;血浆白蛋白水平和抗氧化能力显着下降;以及组织病理学和亚细胞结构的明显变化。与G-CK并用可以缓解所有这些变化。蛋白质组学分析表明,铁调素,可溶性环氧化物水解酶(sEH,UniProt ID P80299)和过氧化物酶体途径参与了G-CK的肝保护作用。分别通过ELISA和Western blot分析验证了hepcidin和sEH蛋白表达的变化。此外,我们观察到SVP组肝铁升高,联合组肝铁降低。总之,我们的研究结果表明,G-CK通过抗氧化作用,过氧化物酶体途径依赖sEH的过氧化调节(P80299)以及铁依赖于铁调素上调的体内稳态,对SVP诱导的肝毒性具有明显的肝保护作用。与G-CK并用可以缓解所有这些变化。蛋白质组学分析表明,铁调素,可溶性环氧化物水解酶(sEH,UniProt ID P80299)和过氧化物酶体途径参与了G-CK的肝保护作用。分别通过ELISA和Western blot分析验证了hepcidin和sEH蛋白表达的变化。此外,我们观察到SVP组肝铁升高,联合组肝铁降低。总而言之,我们的发现证明了G-CK通过抗氧化作用,过氧化物酶体途径依赖sEH(P80299)下调以及铁稳态(依赖于铁调素上调)的调节,对SVP诱导的肝毒性具有明显的肝保护作用。与G-CK并用可以缓解所有这些变化。蛋白质组学分析表明,铁调素,可溶性环氧化物水解酶(sEH,UniProt ID P80299)和过氧化物酶体途径参与了G-CK的肝保护作用。分别通过ELISA和Western blot分析验证了hepcidin和sEH蛋白表达的变化。另外,我们观察到SVP组肝铁升高,联合组肝铁降低。总而言之,我们的发现证明了G-CK通过抗氧化作用,过氧化物酶体途径依赖sEH(P80299)下调以及铁稳态(依赖于铁调素上调)的调节,对SVP诱导的肝毒性具有明显的肝保护作用。可溶性环氧化物水解酶(sEH,UniProt ID P80299)和过氧化物酶体途径参与了G-CK的肝保护作用。分别通过ELISA和Western blot分析验证了hepcidin和sEH蛋白表达的变化。另外,我们观察到SVP组肝铁升高,联合组肝铁降低。总而言之,我们的发现证明了G-CK通过抗氧化作用,过氧化物酶体途径依赖sEH(P80299)的下调以及铁稳态(依赖于铁调素的上调)的调节,对SVP诱导的肝毒性具有明显的肝保护作用。可溶性环氧化物水解酶(sEH,UniProt ID P80299)和过氧化物酶体途径参与了G-CK的肝保护作用。分别通过ELISA和Western blot分析验证了hepcidin和sEH蛋白表达的变化。另外,我们观察到SVP组肝铁升高,联合组肝铁降低。总而言之,我们的发现证明了G-CK通过抗氧化作用,过氧化物酶体途径依赖sEH(P80299)的下调以及铁稳态(依赖于铁调素的上调)的调节,对SVP诱导的肝毒性具有明显的肝保护作用。分别通过ELISA和Western blot分析验证了hepcidin和sEH蛋白表达的变化。另外,我们观察到SVP组肝铁升高,联合组肝铁降低。总而言之,我们的发现证明了G-CK通过抗氧化作用,过氧化物酶体途径依赖sEH(P80299)的下调以及铁稳态(依赖于铁调素的上调)的调节,对SVP诱导的肝毒性具有明显的肝保护作用。分别通过ELISA和Western blot分析验证了hepcidin和sEH蛋白表达的变化。另外,我们观察到SVP组肝铁升高,联合组肝铁降低。总而言之,我们的发现证明了G-CK通过抗氧化作用,过氧化物酶体途径依赖sEH(P80299)的下调以及铁稳态(依赖于铁调素的上调)的调节,对SVP诱导的肝毒性具有明显的肝保护作用。
更新日期:2019-11-14
中文翻译:

人参皂甙化合物K通过抗氧化作用,调节过氧化物酶体途径和铁稳态来减轻丙戊酸钠诱导的大鼠肝毒性。
丙戊酸钠(SVP)是治疗各种形式癫痫的一线治疗药物。但是,它可能导致严重的肝损伤。人参皂苷化合物K(G-CK)是传统草药人参的主要活性成分。根据我们先前的研究,据信G-CK可在LiCl-毛果芸香碱诱发的癫痫大鼠中显着逆转SVP诱导的ALT和AST水平升高以及肝脏组织的病理变化。因此,我们旨在评估G-CK对SVP引起的肝毒性的保护作用。用SVP治疗的大鼠表现出肝损伤,肝指数,转氨酶活性,碱性磷酸酶水平,肝甘油三酸酯和脂质过氧化明显增加;血浆白蛋白水平和抗氧化能力显着下降;以及组织病理学和亚细胞结构的明显变化。与G-CK并用可以缓解所有这些变化。蛋白质组学分析表明,铁调素,可溶性环氧化物水解酶(sEH,UniProt ID P80299)和过氧化物酶体途径参与了G-CK的肝保护作用。分别通过ELISA和Western blot分析验证了hepcidin和sEH蛋白表达的变化。此外,我们观察到SVP组肝铁升高,联合组肝铁降低。总之,我们的研究结果表明,G-CK通过抗氧化作用,过氧化物酶体途径依赖sEH的过氧化调节(P80299)以及铁依赖于铁调素上调的体内稳态,对SVP诱导的肝毒性具有明显的肝保护作用。与G-CK并用可以缓解所有这些变化。蛋白质组学分析表明,铁调素,可溶性环氧化物水解酶(sEH,UniProt ID P80299)和过氧化物酶体途径参与了G-CK的肝保护作用。分别通过ELISA和Western blot分析验证了hepcidin和sEH蛋白表达的变化。此外,我们观察到SVP组肝铁升高,联合组肝铁降低。总而言之,我们的发现证明了G-CK通过抗氧化作用,过氧化物酶体途径依赖sEH(P80299)下调以及铁稳态(依赖于铁调素上调)的调节,对SVP诱导的肝毒性具有明显的肝保护作用。与G-CK并用可以缓解所有这些变化。蛋白质组学分析表明,铁调素,可溶性环氧化物水解酶(sEH,UniProt ID P80299)和过氧化物酶体途径参与了G-CK的肝保护作用。分别通过ELISA和Western blot分析验证了hepcidin和sEH蛋白表达的变化。另外,我们观察到SVP组肝铁升高,联合组肝铁降低。总而言之,我们的发现证明了G-CK通过抗氧化作用,过氧化物酶体途径依赖sEH(P80299)下调以及铁稳态(依赖于铁调素上调)的调节,对SVP诱导的肝毒性具有明显的肝保护作用。可溶性环氧化物水解酶(sEH,UniProt ID P80299)和过氧化物酶体途径参与了G-CK的肝保护作用。分别通过ELISA和Western blot分析验证了hepcidin和sEH蛋白表达的变化。另外,我们观察到SVP组肝铁升高,联合组肝铁降低。总而言之,我们的发现证明了G-CK通过抗氧化作用,过氧化物酶体途径依赖sEH(P80299)的下调以及铁稳态(依赖于铁调素的上调)的调节,对SVP诱导的肝毒性具有明显的肝保护作用。可溶性环氧化物水解酶(sEH,UniProt ID P80299)和过氧化物酶体途径参与了G-CK的肝保护作用。分别通过ELISA和Western blot分析验证了hepcidin和sEH蛋白表达的变化。另外,我们观察到SVP组肝铁升高,联合组肝铁降低。总而言之,我们的发现证明了G-CK通过抗氧化作用,过氧化物酶体途径依赖sEH(P80299)的下调以及铁稳态(依赖于铁调素的上调)的调节,对SVP诱导的肝毒性具有明显的肝保护作用。分别通过ELISA和Western blot分析验证了hepcidin和sEH蛋白表达的变化。另外,我们观察到SVP组肝铁升高,联合组肝铁降低。总而言之,我们的发现证明了G-CK通过抗氧化作用,过氧化物酶体途径依赖sEH(P80299)的下调以及铁稳态(依赖于铁调素的上调)的调节,对SVP诱导的肝毒性具有明显的肝保护作用。分别通过ELISA和Western blot分析验证了hepcidin和sEH蛋白表达的变化。另外,我们观察到SVP组肝铁升高,联合组肝铁降低。总而言之,我们的发现证明了G-CK通过抗氧化作用,过氧化物酶体途径依赖sEH(P80299)的下调以及铁稳态(依赖于铁调素的上调)的调节,对SVP诱导的肝毒性具有明显的肝保护作用。


























 京公网安备 11010802027423号
京公网安备 11010802027423号