当前位置:
X-MOL 学术
›
J. Comput. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
A Minimal Membrane Metal Transport System: Dynamics and Energetics of mer Proteins
Journal of Computational Chemistry ( IF 3 ) Pub Date : 2019-11-13 , DOI: 10.1002/jcc.26098 Hyea Hwang 1, 2 , Anthony Hazel 3 , Peng Lian 2, 4 , Jeremy C Smith 2, 4 , James C Gumbart 3 , Jerry M Parks 2
Journal of Computational Chemistry ( IF 3 ) Pub Date : 2019-11-13 , DOI: 10.1002/jcc.26098 Hyea Hwang 1, 2 , Anthony Hazel 3 , Peng Lian 2, 4 , Jeremy C Smith 2, 4 , James C Gumbart 3 , Jerry M Parks 2
Affiliation
|
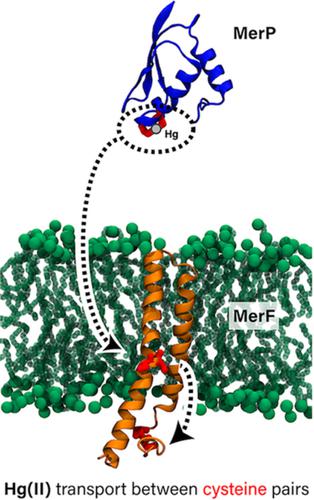
The mer operon in bacteria encodes a set of proteins and enzymes that impart resistance to environmental mercury toxicity by importing Hg2+ and reducing it to volatile Hg(0). Because the reduction occurs in the cytoplasm, mercuric ions must first be transported across the cytoplasmic membrane by one of a few known transporters. MerF is the smallest of these, containing only two transmembrane helices and two pairs of vicinal cysteines that coordinate mercuric ions. In this work, we use molecular dynamics simulations to characterize the dynamics of MerF in its apo and Hg2+‐bound states. We find that the apo state positions one of the cysteine pairs closer to the periplasmic side of the membrane, while in the bound state the same pair approaches the cytoplasmic side. This finding is consistent with the functional requirement of accepting Hg2+ from the periplasmic space, sequestering it on acceptance, and transferring it to the cytoplasm. Conformational changes in the TM helices facilitate the functional interaction of the two cysteine pairs. Free‐energy calculations provide a barrier of 16 kcal/mol for the association of the periplasmic Hg2+‐bound protein MerP with MerF and 7 kcal/mol for the subsequent association of MerF's two cysteine pairs. Despite the significant conformational changes required to move the binding site across the membrane, coarse‐grained simulations of multiple copies of MerF support the expectation that it functions as a monomer. Our results demonstrate how conformational changes and binding thermodynamics could lead to such a small membrane protein acting as an ion transporter. Published 2019. This article is a U.S. Government work and is in the public domain in the USA.
中文翻译:

最小膜金属转运系统:mer 蛋白质的动力学和能量学
细菌中的 mer 操纵子编码一组蛋白质和酶,它们通过导入 Hg2+ 并将其还原为挥发性 Hg(0) 来赋予对环境汞毒性的抵抗力。由于还原发生在细胞质中,汞离子必须首先通过少数已知转运蛋白之一穿过细胞质膜。MerF 是其中最小的,仅包含两个跨膜螺旋和两对协调汞离子的邻位半胱氨酸。在这项工作中,我们使用分子动力学模拟来表征处于 apo 和 Hg2+ 结合状态的 MerF 的动力学。我们发现 apo 状态将其中一个半胱氨酸对定位为更靠近膜的周质侧,而在结合状态下,同一对接近细胞质侧。这一发现与从周质空间接受 Hg2+、在接受时将其隔离并将其转移到细胞质的功能要求是一致的。TM 螺旋的构象变化促进了两个半胱氨酸对的功能相互作用。自由能计算为周质 Hg2+ 结合蛋白 MerP 与 MerF 的结合提供了 16 kcal/mol 的屏障,为随后 MerF 的两个半胱氨酸对的结合提供了 7 kcal/mol 的屏障。尽管跨膜移动结合位点需要显着的构象变化,但对 MerF 多个副本的粗粒度模拟支持其作为单体发挥作用的预期。我们的结果证明了构象变化和结合热力学如何导致如此小的膜蛋白充当离子转运蛋白。
更新日期:2019-11-13
中文翻译:

最小膜金属转运系统:mer 蛋白质的动力学和能量学
细菌中的 mer 操纵子编码一组蛋白质和酶,它们通过导入 Hg2+ 并将其还原为挥发性 Hg(0) 来赋予对环境汞毒性的抵抗力。由于还原发生在细胞质中,汞离子必须首先通过少数已知转运蛋白之一穿过细胞质膜。MerF 是其中最小的,仅包含两个跨膜螺旋和两对协调汞离子的邻位半胱氨酸。在这项工作中,我们使用分子动力学模拟来表征处于 apo 和 Hg2+ 结合状态的 MerF 的动力学。我们发现 apo 状态将其中一个半胱氨酸对定位为更靠近膜的周质侧,而在结合状态下,同一对接近细胞质侧。这一发现与从周质空间接受 Hg2+、在接受时将其隔离并将其转移到细胞质的功能要求是一致的。TM 螺旋的构象变化促进了两个半胱氨酸对的功能相互作用。自由能计算为周质 Hg2+ 结合蛋白 MerP 与 MerF 的结合提供了 16 kcal/mol 的屏障,为随后 MerF 的两个半胱氨酸对的结合提供了 7 kcal/mol 的屏障。尽管跨膜移动结合位点需要显着的构象变化,但对 MerF 多个副本的粗粒度模拟支持其作为单体发挥作用的预期。我们的结果证明了构象变化和结合热力学如何导致如此小的膜蛋白充当离子转运蛋白。



























 京公网安备 11010802027423号
京公网安备 11010802027423号