Cell Host & Microbe ( IF 30.3 ) Pub Date : 2019-11-13 , DOI: 10.1016/j.chom.2019.10.011 Katharine Michelle Ng 1 , Andrés Aranda-Díaz 1 , Carolina Tropini 2 , Matthew Ryan Frankel 1 , William Van Treuren 2 , Colleen T O'Loughlin 1 , Bryan Douglas Merrill 2 , Feiqiao Brian Yu 3 , Kali M Pruss 2 , Rita Almeida Oliveira 4 , Steven Kyle Higginbottom 2 , Norma F Neff 3 , Michael Andrew Fischbach 5 , Karina Bivar Xavier 4 , Justin Laine Sonnenburg 6 , Kerwyn Casey Huang 7
|
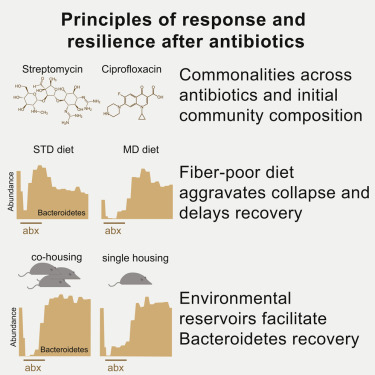
Antibiotics alter microbiota composition and increase infection susceptibility. However, the generalizable effects of antibiotics on and the contribution of environmental variables to gut commensals remain unclear. To address this, we tracked microbiota dynamics with high temporal and taxonomic resolution during antibiotic treatment in a controlled murine system by isolating variables such as diet, treatment history, and housing co-inhabitants. Human microbiotas were remarkably resilient and recovered during antibiotic treatment, with transient dominance of resistant Bacteroides and taxa-asymmetric diversity reduction. In certain cases, in vitro sensitivities were not predictive of in vivo responses, underscoring the significance of host and community context. A fiber-deficient diet exacerbated microbiota collapse and delayed recovery. Species replacement through cross housing after ciprofloxacin treatment established resilience to a second treatment. Single housing drastically disrupted recovery, highlighting the importance of environmental reservoirs. Our findings highlight deterministic microbiota adaptations to perturbations and the translational potential for modulating diet, sanitation, and microbiota composition during antibiotics.
中文翻译:

抗生素使用后肠道菌群的恢复取决于宿主饮食,社区环境和环境贮藏库。
抗生素会改变微生物群的组成并增加感染的易感性。然而,对抗生素的普遍影响以及环境变量对肠道功能的影响尚不清楚。为了解决这个问题,我们通过隔离变量(例如饮食,治疗史和共同居住者),在受控的鼠类系统中以高时间和分类学分辨率跟踪了微生物群落动态,从而进行了抗生素治疗。人类microbiotas均显着的弹性和抗生素治疗过程中回收,用耐瞬态显性杆菌和门类不对称多样性降低。在某些情况下,体外敏感性不能预测体内回应,强调寄主和社区背景的重要性。缺乏纤维的饮食加剧了微生物群的崩溃并延缓了恢复。环丙沙星治疗后通过交叉饲养的物种替代建立了对第二种治疗的适应力。单一房屋严重破坏了恢复,突出了环境水库的重要性。我们的发现强调了确定性微生物群对扰动的适应性以及在抗生素过程中调节饮食,卫生条件和微生物群组成的转化潜力。


























 京公网安备 11010802027423号
京公网安备 11010802027423号